Background and overview[1]
3-Bromo-4-methoxyaniline has been found to have a wide range of uses in medicine. 3-Bromo-4-methoxyaniline can be used to prepare analogs of the anti-cancer drug Combretastatin A-4, drugs that replace cell cycle checkpoint kinase 1 (CHK1), and to treat obesity and the body Disordered drugs, drugs with Src kinase inhibitory activity, drugs that can replace tyrosine kinase inhibitors, drugs that can replace endo-β-glucuronidase heparanase inhibitors, treatment of polycystic kidney disease, colon polyps, Cancer, drugs for mammalian stroke, drugs to inhibit smooth muscle cell proliferation, synthetic sulfonamides with antibacterial properties.
At present, there are two main synthesis methods for 3-bromo-4-methoxyaniline: (1) Using SnCl2·2H2O to reduce 3-bromo-4-methoxynitrobenzene to obtain 3-bromo-4-methyl Oxyaniline, the yield is 67%. The disadvantage is that stannous chloride is relatively expensive, the dosage is large, the reaction cost is high, and it is unstable in the air and is easily oxidized and ineffective. The conversion rate of this reaction is not high, only 67%, so it is not suitable for industrial production. (2) Utilize the reaction between electron-rich o-methoxybromobenzene and electron-deficient (E)-bis(2,2,2-trichloro)azo-1,2-dicarboxylic acid, diethyl ether as the solvent, and BF3 as Catalyst, reaction at room temperature for 8 hours, yield 75%.
The raw materials of this method are chlorine-containing compounds and are expensive. The solvent uses flammable and explosive ether with a low boiling point, which has great safety risks and is difficult to carry out industrial production. In summary, there are currently few reports on the synthesis methods of 3-bromo-4-methoxyaniline, and they are not suitable for industrial production. Therefore, it is necessary to develop a synthetic route suitable for industrial production.
Preparation [1]
Using p-fluoronitrobenzene as raw material, 3-bromo-4-methoxyaniline is synthesized through three steps of bromination, etherification and nitro reduction. This method includes the following reaction equation:
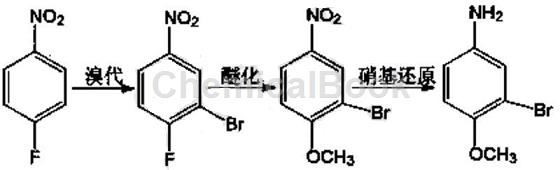
Follow these steps:
(1) Bromination reaction: Add p-fluoronitrobenzene and solvent acetic acid to the reaction vessel, control the temperature to 15-60°C, slowly add the bromination reagent under stirring, after the reaction is completed, pour into ice water, and the product will be The solid is precipitated, filtered, and dried to obtain 3-bromo-4-fluoronitrobenzene, a light yellow powdery solid, in which the ratio of the amount of acetic acid to p-fluoronitrobenzene used ranges from 4.0 to 8.0:1, where The brominated reagent is bromate or bromine or N-bromosuccinimide; the ratio of the amount of the brominated reagent to p-fluoronitrobenzene ranges from 1.0 to 1.2:1. .
The preferred temperature range is 25-50°C, and the optimal temperature range is 30-40°C; the preferred optimal ratio range of the amounts of acetic acid and p-fluoronitrobenzene is 5.0-6.0:1. The ratio of the amounts of the brominated reagent N-bromosuccinimide and p-fluoronitrobenzene ranges from 1.05 to 1.1:1.
(2) Etherification reaction: Add the product 3-bromo-4-fluoronitrobenzene and the solvent methanol in the first step to the reaction vessel, control the temperature at 10~80°C, slowly add sodium methoxide while stirring, where The ratio of the volume of sodium methoxide to 3-bromo-4-fluoronitrobenzene is 3.5 to 5.5:1; the ratio of the volume of solvent methanol to 3-bromo-4-fluoronitrobenzene is 2000 ~6000 ml: 1 mol; after the reaction is completed, pour the product into ice water, precipitate the solid, and filter it with suction to obtain 3-bromo-4-methoxynitrobenzene, a white flocculent solid.
Choose a temperature range of 20 to 60 ℃, and the optimal temperature range is 25 to 40 ℃. The ratio of the amounts of sodium methoxide to 3-bromo-4-fluoronitrobenzene ranges from 4.0 to 5.0:1. The ratio of the volume of solvent methanol to 3-bromo-4-fluoronitrobenzene ranges from 3000 to 4000 ml: 1 mol.
(3) Nitro reduction reaction: Add the product of the second step, 3-bromo-4-methoxynitrobenzene, into the reaction vessel, add water to 1/2 of the reaction vessel, and control the temperature at 70 to 100°C , after the temperature reaches the temperature range, start adding the reducing agent, where the ratio of the amount of reducing agent to 3-bromo-4-methoxynitrobenzene ranges from 3.0 to 6.0. After the reaction is completed, pour the reaction product into ice water , the solid is precipitated and filtered to obtain a yellow powdery solid, in which the reducing agent is Na2S or iron powder or ammonium chloride. The preferred reaction temperature range is 85-95°C, and the optimal temperature range is 90-95°C. The reducing agent described therein��Na2S, the ratio of the amount of the substance to 3-bromo-4-methoxynitrobenzene ranges from 4.0 to 5.0.
The specific implementation is as follows:
1) Bromination reaction: Add 7.05 g of p-fluoronitrobenzene and 25 g of acetic acid into the reaction vessel, and control the temperature of the water bath to 15°C. Slowly add 9.34 g N-bromosuccinimide while stirring. During the addition process, the temperature should not exceed 15°C. After the addition, the reaction was kept warm for 10 h, and the reaction was completed. After the reaction, the reactant was poured into 500 ml of ice water, the solid was precipitated, filtered, and dried to obtain 9.95 g of 3-bromo-4-fluoronitrobenzene as a light yellow powdery solid, with a yield of 90.5%.
2) Etherification reaction: Add 11.0 g 3-bromo-4-fluoronitrobenzene and 175 ml methanol into the reaction vessel. Control the water bath temperature at 10°C. Slowly add 12.15 g sodium methoxide. During the addition process, the system temperature Maintain at 10°C. After the addition was completed, the reaction was kept warm for 4 h, and the reaction was completed. After the reaction, the reactant was poured into 1000 ml of ice water, the solid was precipitated, filtered, and dried to obtain 11.15 g of white flocculent solid 3-bromo-4-methoxynitrobenzene, with a yield of 96.1%.
3) Nitro reduction reaction: Add 11.6 g of 3-bromo-4-methoxynitrobenzene into the reaction vessel, add water to 1/2 of the reaction vessel, raise the temperature to 70°C, and add 17.55 g in batches After the addition of Na2S, the reaction was incubated for 10 h and the reaction was completed. After the reaction, the reactant was poured into 2000 ml ice water, the solid was precipitated, filtered, and dried to obtain 7.2 g of yellow powdery solid 3-bromo-4-methoxyaniline, with a yield of 71.3%.
Main reference materials
[1] CN201110089719.3 Preparation method of 3-bromo-4-methoxyaniline



 微信扫一扫打赏
微信扫一扫打赏
