Background and overview[1][2]
3 Cyanobenzoic acid is an important organic synthesis intermediate. It is widely used in the preparation of cardiovascular drugs, antibiotics, analgesics and nervous system regulating drugs, etc. It is also an important raw material for the synthesis of liquid crystal materials. The existing synthesis methods of 3-cyanobenzoic acid mainly include the following:
1) Using 3-cyanotoluene as raw material, divalent cobalt salt as catalyst, sodium bromide as cocatalyst, and oxygen in the air as oxidant, prepare 3-cyanobenzoic acid with a yield of 80-99%. The disadvantage of this method is that the reaction needs to be carried out in a sealed tube, with high reaction temperature and high pressure. This method is particularly suitable for the preparation of 3-cyanobenzoic acid in small batches in chemistry laboratories.
2) Using 3-cyanotoluene as the raw material, acetic acid as the solvent, chromium trioxide as the oxidant, and sulfuric acid as the cocatalyst, the product is oxidized at a temperature of 0-5°C and undergoes a series of post-processing processes to obtain the product. The process is simple and the production cost is low. However, excessive chromium trioxide treatment is dangerous and can easily cause fires and explosions. The treatment of large amounts of trivalent chromium wastewater is also a big problem, so it is only suitable for the preparation of 3-cyanobenzoic acid in small batches.
3) Using 1,3 phthalonitrile as the starting material, through selective hydrolysis of methanol/water/alkali, 3-cyanobenzoic acid is obtained with a yield of about 75%. This reaction is not easy to control the single hydrolysis of two CNs, so the content of the final product is not high, there are many three wastes, and it is not suitable for large-scale preparation of 3-cyanobenzoic acid.
4) Use lead acetate and fluoropolyether as catalysts, add carbon monoxide to the dimethyl sulfoxide solution of 3 chlorobenzonitrile at normal pressure, and react at 100°C for 15 hours to obtain 3 cyanobenzoic acid. The synthesis yield using this method is very high, up to 97%. However, this method involves more substances participating in the reaction, the reaction process is more complicated, and the operation is inconvenient.
Apply[3]
The functional groups in the structure of 3-cyanobenzoic acid (methyl ester) have different activities. It is a prerequisite compound for the synthesis of a series of medicines, pesticides, fluorescent whitening agents, high-end liquid crystal materials, functional polymer materials, etc.
1. Application in medicine
3-Cyanobenzoic acid can be used to prepare new drugs L-779976 and L-054264 for the treatment of digestive tract tumor diseases. PTC124, synthesized from methyl 3-cyanobenzoate, can be used to treat Duchenne muscular dystrophy and cystic fibrosis. Digestive tract diseases are a very common disease in daily life. In recent years, with the deterioration of food safety and the increase in people’s life pressure, this disease that originally occurred in the elderly has become younger and more serious.
According to a 2011 report in the magazine “Chinese Oncology”, China has become an area with a high incidence of digestive tract diseases in the world. Take gastric cancer as an example. According to statistics from the Chinese Society of Clinical Oncology, the incidence of gastric cancer in China accounts for as high as 42% in the world. Hundreds of thousands of people are diagnosed with the disease every year, and the number of deaths accounts for two-thirds of the number of cases. The number is close to three hundred thousand. Digestive tract tumor disease has become one of the common high-risk diseases, with many complications and high mortality, and it is still a major clinical problem. Somatostatin (SS) is a growth hormone release control factor that was first isolated and extracted from sheep hypothalamus by American researcher Brazo. It mainly exists in normal tissues of the human body and has regulatory and inhibitory physiological effects. It can be used for the diagnosis and treatment of gastrointestinal tumor diseases with good results.
Due to the universal physiological functions of SS and its close relationship with the occurrence, development and treatment of many tumor diseases, researchers have paid much attention to its medical application value. The new drug L-779976 and the new drug L-054264 synthesized by Merck Company in the United States are both artificially produced somatostatin and can be used to treat digestive tract tumor diseases. The starting material for the synthesis of both new drugs is 3-cyanobenzoic acid. The American company Merck purchases part of 3-cyanobenzoic acid through third parties every year for clinical research. Taking the new drug L-054264 as an example, the synthesis route map is as follows:
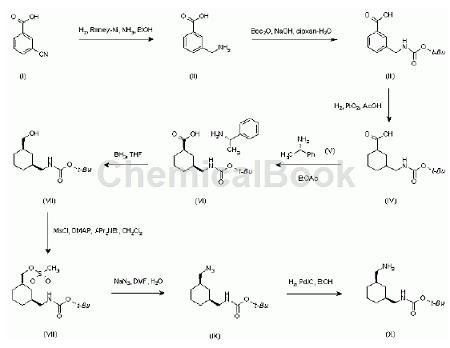
2. Application in functional materials
Functional materials are new materials that developed rapidly in the late 1960s. “Function” means that in addition to mechanical properties, this new type of material may also have optical, electrical, magnetic and other properties, which are all similar to functional materials.Special functional groups are closely related. Functional materials have been widely used in many fields. Biomedical functional materials and environmental treatment functional materials are widely used. The unique functional properties of functional materials have contributed to technological progress in many fields, even caused qualitative leaps in some fields, created high economic and social benefits in various industries, and produced many useful new products.
As people’s research on functional materials gradually deepens and strengthens, there are currently two research directions for functional materials: some researchers improve traditional polymer materials to continuously improve their performance while increasing Its functional application range; there are also researchers directly engaged in research in the field of new materials that are closely related to humans and have special functions, such as bionic materials. Functional materials are developing rapidly in the fields of materials science and engineering technology in the future, which will have great significance for human production and life. Some researchers use the trimerization of 3-cyanobenzoic acid to synthesize intermediates for high-temperature resistant materials.
Connect 3-cyanobenzoic acid to the porphyrin ring to obtain 5-(4-3-cyanobenzoic acid imide)phenyl-10,15,20-triphenylporphyrin ligand (CBTPPH2), this new ligand has active superposition and is an important functional material. Its synthesis route is as follows:
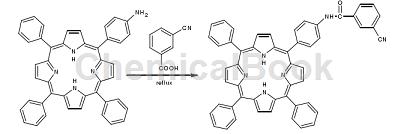
Preparation [1-2]
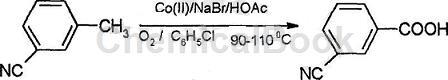
Method 1: A method for preparing m-cyanobenzoic acid with simple process and low production cost. This purpose is achieved by using the following process: m-cyanotoluene is used as raw material, chlorobenzene is used as solvent; cobalt acetate is used as catalyst, sodium bromide and glacial acetic acid are used as cocatalysts, the reaction liquid is mixed and then passed into Excess oxygen is oxidized in one step and separated to obtain the product. Preparation steps:
Step 1: Prepare the reaction solution. Weigh 100 parts of m-cyanotoluene, 4.26 to 20.2 parts of cobalt acetate, 2.03 to 5.15 parts of sodium bromide and 20 to 40 parts of 99% glacial acetic acid, and dissolve them in 412 parts of chlorobenzene. Prepare the reaction solution;
Step 2: Oxidation, stir the reaction solution prepared in the previous step for 3 to 10 minutes at a temperature and pressure of 90 to 110°C and 1 atmosphere respectively, and then pass excess oxygen into the reaction solution. The flow rate is 4~5L/h, and the reaction is 3.5~7h;
Step 3: Separate, cool the oxidized reaction solution in the previous step to room temperature, add 450 to 650 parts of water, there is a white lamellar solid in the reaction solution, filter, take the solid, heat and dry in the air to obtain the product , the yield is 80~99%.
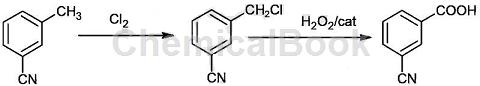
Method 2:3 Production method of cyanobenzoic acid, the reaction is carried out in two steps:
In the first step, add 800 liters of 3-methylbenzonitrile into the 1000-liter enamel reactor, heat it to 25-160°C, and use a pump to continuously pump the liquid into the top of the packed tower at a rate of 5-40 liters/ Minutes flow rate is sprayed downward, and at the same time, 310 kilograms of chlorine gas is evenly passed upward from the bottom of the packed tower at a flow rate of 5 to 40 liters/minute to form 3 methyl benzonitrile and chlorine gas in reverse motion for chlorination reaction; the gas phase passes through the chip enamel The condenser is cooled, the gas-liquid separator separates, and the liquid flows back into the enamel reaction kettle; hydrogen chloride and chlorine gas enter the tail gas absorption tank and are absorbed by water and alkali and then emptied; after the reaction is completed, the material liquid is transferred to the enamel crystallization kettle and cooled to 5 ℃, suction filtration, the filter cake is 3 chloromethylbenzonitrile;
In the second step, add 200kg of 3-chloromethylbenzonitrile, 100-800kg of ethanol, 13kg of catalyst and 0.21kg of benzyltriethylammonium chloride salt phase transfer catalyst into 2000 liters of enamel reaction In the kettle, stir and raise the temperature; keep the reaction temperature at 30-50°C, and slowly add 150-450 kg of 30% hydrogen peroxide into the reaction kettle. After the dropwise addition is completed, the temperature is maintained for 6 hours. The oxidation reaction generates 3-cyanobenzoic acid. Continue heating to 100°C to distill out ethanol. Cool to room temperature, filter, wash the filter cake twice with water, recrystallize with ethanol, and dry to obtain 3-cyanobenzoic acid white powder;
The catalyst is vanadium sulfate and sodium tungstate, the mass ratio is 1:10 10:1; the dosage of the catalyst is 0.5 1.5% of the mass of 3 chloromethyl benzonitrile; the 3 methyl chloride The molar ratio of benzyl benzonitrile to hydrogen peroxide is 1:1 3, the amount of benzyl triethylammonium chloride phase transfer catalyst is 0.1 0.5% of the mass of 3 chloromethyl benzonitrile, the oxidation reaction is 3 chloromethyl The mass ratio of benzonitrile to ethanol is 1:0.5 4;
The specific reaction formula is as follows:
Main reference materials
[1] CN01105687.8 A method for preparing m-cyanobenzoic acid
[2] CN01105687.8 A method for preparing m-cyanobenzoic acid
[3] Research on the synthesis process of 3-cyanobenzoic acid (methyl ester)
�Enter the tail gas absorption tank, absorb water and alkali, and then vent it; after the reaction is completed, transfer the material liquid to the enamel crystallization kettle, cool it to 5°C, and filter it with suction. The filter cake is 3 chloromethyl benzonitrile;
In the second step, add 200kg of 3-chloromethylbenzonitrile, 100-800kg of ethanol, 13kg of catalyst and 0.21kg of benzyltriethylammonium chloride salt phase transfer catalyst into 2000 liters of enamel reaction In the kettle, stir and raise the temperature; keep the reaction temperature at 30-50°C, and slowly add 150-450 kg of 30% hydrogen peroxide into the reaction kettle. After the dropwise addition is completed, the temperature is maintained for 6 hours. The oxidation reaction generates 3-cyanobenzoic acid. Continue heating to 100°C to distill out ethanol. Cool to room temperature, filter, wash the filter cake twice with water, recrystallize with ethanol, and dry to obtain 3-cyanobenzoic acid white powder;
The catalyst is vanadium sulfate and sodium tungstate, the mass ratio is 1:10 10:1; the dosage of the catalyst is 0.5 1.5% of the mass of 3 chloromethyl benzonitrile; the 3 methyl chloride The molar ratio of benzyl benzonitrile to hydrogen peroxide is 1:1 3, the amount of benzyl triethylammonium chloride phase transfer catalyst is 0.1 0.5% of the mass of 3 chloromethyl benzonitrile, the oxidation reaction is 3 chloromethyl The mass ratio of benzonitrile to ethanol is 1:0.5 4;
The specific reaction formula is as follows:

Main reference materials
[1] CN01105687.8 A method for preparing m-cyanobenzoic acid
[2] CN01105687.8 A method for preparing m-cyanobenzoic acid
[3] Research on the synthesis process of 3-cyanobenzoic acid (methyl ester)



 微信扫一扫打赏
微信扫一扫打赏
