Overview
Phthalic anhydride, the full name is phthalic anhydride (Phthalic annychide), the English abbreviation is PA, is a cyclic anhydride formed by intramolecular dehydration of phthalic acid. Easily soluble in hot water, it dissolves into phthalic acid in hot water; it is also soluble in alcohol; it is insoluble in carbon disulfide; it is slightly soluble in cold water, and it is a white needle-like crystal at room temperature (industrial phthalic anhydride is a white flake crystal). It is flammable, easily sublimates below the boiling point, and has a special, slightly pungent odor. Phthalic anhydride can cause allergic symptoms in people’s respiratory organs. The dust or vapor of phthalic anhydride can irritate the skin, eyes and respiratory tract, especially moist tissues.
Phthalic anhydride is mainly used in the production of PVC plasticizers, unsaturated polyesters, alkyd resins, dyes, coatings, pesticides, pharmaceutical and instrument additives, edible saccharin, etc. It is an important organic chemical raw material. In PVC production, the maximum amount of plasticizer used has exceeded 50%. With the rapid development of the plastics industry, the demand for phthalic anhydride has increased, promoting the rapid development of phthalic anhydride production at home and abroad.
The earliest production of phthalic anhydride began in 1872. At that time, the German BASF company used naphthalene as raw material and oxidized it with chromic acid to produce phthalic anhydride. Later, it switched to using oleum oxidation to produce phthalic anhydride, but the yield was extremely low, only 15%[1]. (Zhao Rendian, Kim Changrye, et al. Aromatic Hydrocarbon Engineering) Since the world began to use vanadium oxide as a catalyst and naphthalene to produce phthalic anhydride in 1917, the production of phthalic anhydride has gradually moved toward industrialization and scale, and has formed two relatively mature methods: the naphthalene method and the o-method. craftsmanship.
Production process[2]
Method 1: Naphthalene method. The reaction principle is that naphthalene and air are oxidized in the gas phase under the action of a catalyst to generate phthalic anhydride.
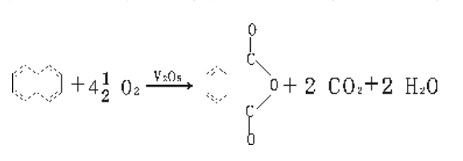
The process flow is that after the air is purified, compressed and preheated, it enters the bottom of the fluidized bed reactor and is injected into liquid naphthalene. After the naphthalene is vaporized, it is mixed with air and passes through the catalyst layer in a fluidized state, where an exothermic reaction occurs to generate phthalic anhydride. The reactor is equipped with a tubular cooler, and water is used as the heat carrier to remove the reaction heat. The reaction gas passes through the three-stage cyclone separator to separate the catalyst carried by the gas, and then enters the liquid condenser. 40%-60% of the crude phthalic anhydride is condensed in liquid state, and the gas then enters the switching condenser (also known as the hot melt box) The crude phthalic anhydride is further separated, and the crude phthalic anhydride is pre-decomposed and then distilled to obtain the finished product. The exhaust gas is washed and discharged, and the washing liquid is diluted with water and then discharged or sent for catalytic incineration. The process flow is as follows:
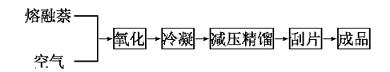
Overview of the naphthalene method: As the earliest method to produce phthalic anhydride, the naphthalene method is also the earliest method to form industrial production. Its raw material is tar naphthalene. my country began to produce phthalic anhydride by naphthalene method in 1953. At that time, naphthalene was used as raw material and fixed bed gas phase oxidation method was used to produce phthalic anhydride. In 1958, my country developed the fluidized bed process and built multiple industrial production units on this basis.
In the production process, the technology has been continuously improved, such as the feeding method has been developed into atomized naphthalene, and the domestic high-efficiency wear-resistant cyclone separator is used for gas-solid separation. These improvements not only greatly improve the output and quality of the product, but also greatly Reduced naphthalene�’s energy consumption. Due to the rapid development of the naphthalene fluidized bed method in my country, most factories were still using the naphthalene fluidized bed method to produce phthalic anhydride by 1988. At that time, the output of the naphthalene method was as high as 90% of the total output. With the development of my country’s petroleum industry and the development of adjacent process technology, the disadvantages of the naphthalene process have emerged: the supply of raw material tar naphthalene is becoming increasingly tight, prices continue to rise, and the production capacity of a single reactor is low. These have inevitably caused the naphthalene process to high energy consumption.
Since naphthalene production has not made any major progress in reducing energy consumption, and under the impact of a large amount of low-priced o-phthalic anhydride, the profit margin of the naphthalene method has become smaller and smaller. In order to improve its own economic benefits, many The naphthalene process manufacturers began to carry out process transformation and turned to the adjacent process production. By 1999, the output of the naphthalene process was less than 10%. By the 21st century, the naphthalene process has been gradually eliminated in my country.
Method 2: Ortho method. The reaction principle is that o-xylene and air are oxidized in the gas phase under the action of a catalyst to generate phthalic anhydride.
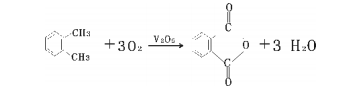
The specific process flow is: the filtered and purified air is compressed, preheated and mixed with vaporized o-xylene into the fixed bed reactor for exothermic reaction. Circulating molten salt is used outside the reaction tube to remove the reaction heat and maintain the reaction. Temperature, the reaction heat brought out by molten salt is used to produce high-pressure steam (high-pressure steam can be used in other aspects of production and can also be used to generate electricity).
The gas from the reactor passes through the precooler and enters the switching condenser that passes cold oil through the fin tube to condense phthalic anhydride on the fins. Then, hot oil is periodically introduced to melt the phthalic anhydride. After heat treatment, it is sent to continuous distillation. The system removes impurities with low boiling point and high swelling point to obtain the finished product of phthalic anhydride. The exhaust gas from the switching condenser is discharged into the atmosphere after two stages of high-efficiency washing. The circulating liquid containing an organic acid concentration of 30% is sent to the maleic anhydride recovery device or incineration device, and can also be recycled and processed to produce fumaric acid. The process flow chart is shown below.
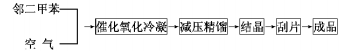
Overview of the o-xylene process: With the rapid growth of phthalic anhydride production, tar naphthalene is increasingly unable to meet production needs. With the development of the petroleum industry, a large amount of cheap o-xylene has been provided, expanding the source of raw materials for phthalic anhydride. . In 1964, the United States used o-xylene as the raw material for gas phase oxidation to produce phthalic anhydride for the first time in industry. Since petroleum o-xylene resources are relatively abundant, the theoretical yield is high [the theoretical yield of phthalic anhydride produced from o-xylene is 139.6 (% mass), and that of naphthalene is 115.6 (% mass)].
Beginning in the 1960s, the raw material for the production of phthalic anhydride was switched from naphthalene to o-xylene. With the significant progress in catalyst research and development and the reduction of the proportion of air and o-xylene participating in the reaction, coupled with the development and application of a series of new technologies such as the realization of large-scale production equipment, the conversion process of raw materials has been further accelerated. Since the 1980s, countries around the world have successively developed “70 g technology”, “80 g technology”, and “90 g technology” and are moving towards higher load technologies. In the 1970s, my country developed and built an industrial unit for the production of phthalic anhydride using the “40 g process” fixed-bed gas phase catalytic oxidation method using o-xylene as raw material. In the 1980s, it introduced two sets of 40,000 t/a and one set of 20,000 t/a from Germany. 10,000 t/a o-method phthalic anhydride production equipment.
The production of phthalic anhydride in my country has gradually shifted to the o-method, and the naphthalene method has been gradually eliminated. In 1992, the domestic production capacity of phthalic anhydride reached 259,500 tons, with the ortho-method accounting for more than 60%. In 1999, the production capacity was nearly 400,000 tons, with the ortho-method accounting for more than 90%. In 2003, the production capacity of phthalic anhydride was approximately 765,000 tons, almost all of which was At this time, there are more than 40 manufacturers of adjacent products, and new adjacent devices are constantly being built and put into production. In recent years, various manufacturers have been continuously improving and perfecting their own processes in terms of energy saving and consumption reduction in order to improve the competitiveness of their products, which makes the adjacent process more mature and advanced.
Production use
Phthalic anhydride is currently widely used in chemical, pharmaceutical, electronics, agriculture, coatings, fine chemicals and other industrial sectors. my country’s phthalic anhydride is mainly used to produce phthalic acid ester plasticizers. The phthalic anhydride consumed accounts for about 60% of the total phthalic anhydride consumption, dyes and paints account for 25%, and unsaturated resins and other products account for about 15%. Phthalic anhydride is an important organic chemical raw material, mainly used in the production of plastic plasticizers, alkyd resins, dyes, unsaturated resins and some medicines and pesticides.
References
[1] Zhao Rendian, Jin Zhangli, et al. Aromatic Hydrocarbon Engineering [M]. Beijing: Chemical Industry Press, 2001.
[2]Ma Weimian. Progress in phthalic anhydride production technology[J]. Coal and Chemical Industry, 2006, 29(9):21-22.
�Introduced two sets of 40,000 t/a and one set of 20,000 t/a ortho-phthalic anhydride production units from Germany.
The production of phthalic anhydride in my country has gradually shifted to the o-method, and the naphthalene method has been gradually eliminated. In 1992, the domestic production capacity of phthalic anhydride reached 259,500 tons, with the ortho-method accounting for more than 60%. In 1999, the production capacity was nearly 400,000 tons, with the ortho-method accounting for more than 90%. In 2003, the production capacity of phthalic anhydride was approximately 765,000 tons, almost all of which was At this time, there are more than 40 manufacturers of adjacent products, and new adjacent devices are constantly being built and put into production. In recent years, various manufacturers have been continuously improving and perfecting their own processes in terms of energy saving and consumption reduction in order to improve the competitiveness of their products, which makes the adjacent process more mature and advanced.
Production use
Phthalic anhydride is currently widely used in chemical, pharmaceutical, electronics, agriculture, coatings, fine chemicals and other industrial sectors. my country’s phthalic anhydride is mainly used to produce phthalic acid ester plasticizers. The phthalic anhydride consumed accounts for about 60% of the total phthalic anhydride consumption, dyes and paints account for 25%, and unsaturated resins and other products account for about 15%. Phthalic anhydride is an important organic chemical raw material, mainly used in the production of plastic plasticizers, alkyd resins, dyes, unsaturated resins and some medicines and pesticides.
References
[1] Zhao Rendian, Jin Zhangli, et al. Aromatic Hydrocarbon Engineering [M]. Beijing: Chemical Industry Press, 2001.
[2]Ma Weimian. Progress in phthalic anhydride production technology[J]. Coal and Chemical Industry, 2006, 29(9):21-22.



 微信扫一扫打赏
微信扫一扫打赏
