Overview【1】【2】
The refined raw material of mesitylene mainly comes from the xylene bottom oil of the platinum reforming unit of the refinery. The bottom oil contains aromatic and non-aromatic components from C5 to C10. There are more than 20 sets of catalytic reforming units in China. About 120,000 tons of heavy aromatics can be produced every year. However, they have not yet been fully separated and utilized. Most of them are mixed with gasoline or burned as fuel, which wastes precious resources. In recent years, with the development of deep processing of C9 aromatics, research on C9 separation has also been in-depth. Domestic research on the synthesis and separation of mesitylene has only begun in the late 1980s. Not only is the device scale small and the purity low, but the method is single and the utilization rate is low. There is an urgent need to develop new production methods. In industrial production, except for mesitylene and o-methylethylbenzene, other C9 aromatic hydrocarbons can be refined by precision distillation.
Since the boiling point difference between mesitylene and o-methylethylbenzene is only 0.4℃, the relative volatility is 1.009. Therefore, domestic and foreign researchers have proposed various separation methods, mainly including the following: isomerization of mesitylene, heavy aromatic distillation separation method, extractive distillation method, molecular sieve adsorption separation method, coordination separation method, and alkylation separation. method, mesitylene gas phase isomerization method (including mesitylene gas liquid phase isomerization, mixed mesitylene gas phase isomerization) and mesitylene atmospheric pressure liquid phase alkylation, etc. Due to the different separation sequences and separation methods of various isomers, there are great differences between various purification methods. Colorless and transparent liquid, insoluble in water, soluble in ethanol, and soluble in benzene, ether and acetone in any proportion. The physical properties of mesitylene are as follows:
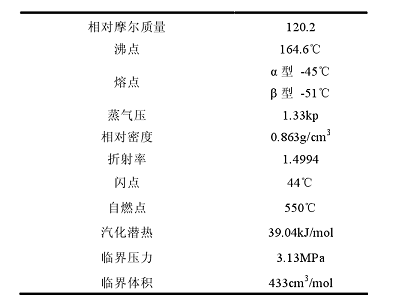
Mesitylene can undergo alkylation, sulfonation, nitration, condensation and other reactions. Mesitylene is a second-level flammable liquid, hazard code number: 62047. Its toxicity is roughly the same as that of xylene. The symptoms of acute poisoning are irritation of mucous membranes and central nervous system; chronic poisoning can cause central nervous system disorders, skin hemorrhagic anemia, and bronchitis. and pulmonary edema, etc.
Purpose【3】
1. Production of dyes
(1) Production of Reactive Brilliant Blue K-3R
Mesitylene undergoes a series of reactions such as sulfonation, nitration and reduction of iron powder to form aromatic amines. Aromatic amines and bromic acid are condensed with cuprous chloride to form a blue radical, which is then activated with ethoxydichloro-s-triazine to form reactive brilliant blue K-3R. K-3R is a new type of dye with bright colors. It is mainly used for printing and tie-dying of cellulose fibers. It is also used for reactive/dispersion process printing and dyeing. It is widely used abroad. In the past, mesitylene used to synthesize K-3R was imported from abroad. With the rapid development of polyester fiber in my country, the demand for mesitylene is also increasing.
(2) Production of weak acid dyes
RAW Mesitylene undergoes a series of reactions such as nitration, neutralization, and reduction to generate mesitylene, which can be synthesized as an intermediate of Prayan blue RAW. This is a weak acid reactive dye with excellent performance, which can make wool or Synthetic fiber products are brightly colored and full of color, and have excellent light resistance, heat resistance and process fastness resistance. In the past, my country’s RAW was entirely imported, with an annual output of about 30t and a price as high as 136,000 yuan/t. Currently, Ruian, Shanghai, Wujiang, Tianjin, Ningbo, Dandong and other places can produce it themselves.
2. Production of pesticides
The wheat field weeding agent mesitylene is nitrated and reduced to form 2,4,6-trimethylaromatic amine, which is then condensed with α-methyl chloropropionate. When used, it is prepared into a 20% solution, per mu The dosage is 0.5kg. Fushun Pesticide Factory is studying this topic. If this product is successfully developed, a large amount of mesitylene will be needed. The current price of similar herbicides (20% solution) is about 3,800 yuan/t.
3. Production of plastics and material intermediates
(1) Antioxidant Lonox-330 The condensation of mesitylene and 2,6 tert-butyl-4(methoxy)phenol in the presence of an acidic catalyst can give 2,4,6-tris(3,5 —Di-tert-butyl-4-hydroxy) mesitylene, trade name Lonox-330, is a highly efficient, non-toxic, heat-resistant, aging-resistant, low-volatility polyphenol antioxidant. Lonox-330 is a white crystalline powder, soluble in benzene and methyl chloride, insoluble in water, stable at high temperatures, low volatility, non-polluting, non-toxic when taken orally, and can be used for plastic products in contact with food.
Lonox-330 can also be used as polyethylene, polyester, polystyrene, synthetic rubber, high melting point lubricants and high temperature adhesives.��Antioxidants and heat stabilizers. The American Ethyl Company sells it for US$17.29/Kg, and this product has been developed in China.
(2) Production of trimesic acid. Mesitylene can be used to produce trimesic acid through liquid phase air oxidation, which can be used to produce new high-temperature engineering plastics. Trimesic acid has three extremely symmetrical functional groups and is an ideal polymer synthetic material. It can be used as raw material for the production of alkyd resin, plasticizer, unsaturated polyester, modified fiber, film, etc. Both the American Amoco Company and Japan’s Mitsubishi Gas Chemical Company can produce this product.
(3) Use sulfuric acid as a catalyst to synthesize various resins. The condensation of mesitylene and formaldehyde can produce light yellow mesitylene-formaldehyde resin. The polymerization of mesitylene, formaldehyde and phenol can synthesize a variety of resins with different properties and uses, which can be used to manufacture electrical insulating paper, printed circuit laminates, PVC, unsaturated resins, polyester resin plasticizers, and can also be used to prepare asphalt. Modifiers and pressure-sensitive adhesives.
4. Production of 3,5-dimethylbenzoic acid
The melting point of 3,5-dimethylbenzoic acid is 172℃~174℃. It can be used to increase the hardening speed of polyurethane, shorten the demoulding time, synthesize prostaglandins, and react with amines to synthesize flame retardants. In addition, mesitylene can also be used to synthesize urethane coatings, epoxy resin curing agents, and can also be directly used as a special solvent. In summary, mesitylene is widely used and is an important raw material for the production of reactive brilliant blue K-3R, weakly acidic dye RAW, antioxidants, and trimesic acid. It is widely used in electronics, printing and dyeing, machinery manufacturing, aerospace, and agriculture. production and other departments.
Overview of research progress at home and abroad 【3】
Benzene and polymethylbenzenes are in increasing demand in industries such as plastics and synthetic fibers. How to reasonably prepare benzene, xylene, mesitylene and mesitylene is a very important topic. Although research on the synthesis and separation of mesitylene has been carried out at home and abroad for a long time, its output has not been high, and there have been relatively few reports on industrial devices. In my country, mesitylene was synthesized using acetone in the early days. In the 1970s, the Taiyuan Institute of Coal Chemistry, Chinese Academy of Sciences, used molecular sieves as catalysts to conduct experiments in the laboratory to produce mesitylene from reformed heavy aromatics using an adsorption separation method. Shanghai Coking once used coal tar as raw material to produce mesitylene reagent in small batches by the Juanization method.
In the late 1980s, the Institute of Petrochemistry of the Heilongjiang Academy of Sciences proposed a process for producing mesitylene through liquid-phase isomerization under normal pressure using mesitylene as raw material. However, due to the need for separation of catalysts and Processes such as water washing and alkylation are intermittent operations and have not been industrialized. In addition, Nanjing Refinery used the isomerization method of mesitylene to produce mesitylene in 1982. Tianjin University cooperated with Hebei Langfang Petrochemical Plant to build an industrial test device with a monthly output of 20 tons of mesitylene in 1997.
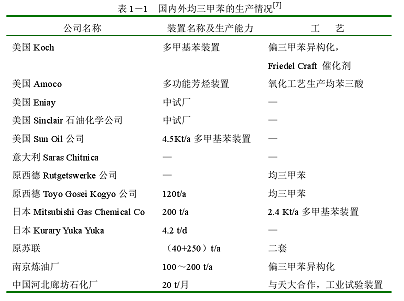
For the production of mesitylene abroad, most of the industrialized devices are directly separated from reformed heavy aromatics. It is said that Japan’s Mitsubishi Gas Chemical Company is the world’s largest producer of mesitylene, with a production capacity of 200t/a. The production status of mesitylene at home and abroad is shown in Table 1-1.
References
[1]Chen Yanjie. Research on mesitylene production technology[D]. Tianjin University, 2004.
[2] Yang Shengkai, Gu Zhenggui. Progress in refining mesitylene[J]. Chemical Engineering Times, 2003(09):1-3.
[3]Wang Shenjiang. Research on the process of synthesizing mesitylene through non-hydrogen isomerization [D]. Tianjin University, 2007.



 微信扫一扫打赏
微信扫一扫打赏
