Background and overview[1][2]
P-Bromotoluene is also known as 4-bromotoluene and 1-bromo-4-methylbenzene. It is a colorless liquid at room temperature, insoluble in water, and soluble in ethanol and ether. Para-bromotoluene is a toxic chemical, harmful if swallowed, and irritating to the eyes, respiratory system and skin. Combustible by open flame. 4-Bromotoluene is an organic synthetic raw material that is widely used in the fuel and pharmaceutical synthesis industries. It is an intermediate for the manufacture of 4-bromobenzyl, 4-bromodibromobenzyl and 4-bromobenzaldehyde. High-purity p-bromotoluene is an intermediate of the important best-selling drug – the anti-hypertensive drug Losantan.
Preparation[1]
The synthesis of p-bromotoluene mainly includes two routes: bromination of toluene and diazotization of p-toluidine. The bromination route of toluene is mostly catalytic bromination. The conditions are harsh and difficult to control. Many isomer by-products are obtained, which are difficult to separate by distillation, and the industrial treatment of bromine is troublesome. At present, the diazotization reaction of p-toluidine is commonly used in industry to produce p-bromotoluene. Using p-toluidine as raw material, the purity of the product after diazotization, synthesis and distillation can reach 99%, but the product yield The rate is 60% to 80%, and the product yield is not ideal. CN201310495723.9 provides a synthesis process of p-bromotoluene, which improves the traditional process to achieve the purpose of increasing product yield. The method includes the following steps: adding p-toluidine and hydrochloric acid to a reaction pot equipped with a stirrer, a condenser, a thermometer and a dropping funnel, stirring and heating to 50-60°C to dissolve the p-toluidine, and cooling to 10-12°C. ; Add sodium nitrite solution dropwise, check with starch potassium iodide test paper to reach the end point and continue stirring for 30 minutes; add copper bromide hydrobromic acid solution to the reaction solution, stir for 2 hours at 25~30°C, and then heat the reaction solution to 70~ 80°C and keep it at this temperature for 0.5h; add steam to the reaction solution for distillation, collect the distillate, use a separatory funnel to separate the p-bromotoluene layer, distill the product under reduced pressure, and collect the fractions at 104-106°C The white crystal obtained is p-bromotoluene. In the synthesis process of p-bromotoluene of the present invention, excess hydrochloric acid is used in the diazotization process to avoid the occurrence of by-products in the diazo process, and cuprous bromide is more likely to react with diazonium salts under the protection of hydrobromic acid. , the reaction efficiency is high, the reaction speed is fast, the product yield reaches 97.82%, and the purity reaches 99.99%.
Storage and transportation equipment[2]
Existing storage and transportation devices for p-bromotoluene are prone to decompose p-bromotoluene to produce toxic gases during the storage and transportation process. Moreover, the structures of these storage and transportation devices are complex and the production costs are high, which is not conducive to the processing of large amounts of p-bromotoluene. Storage and transportation.
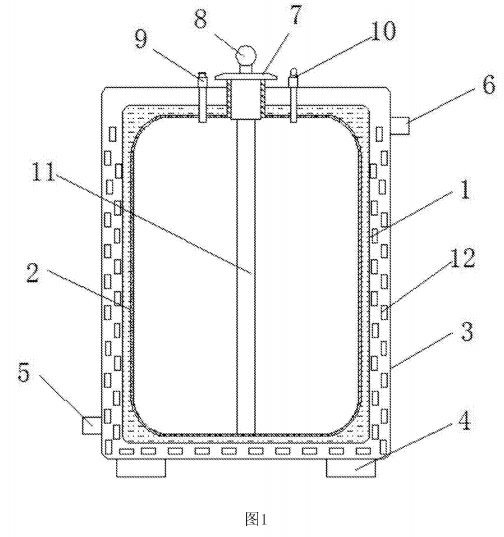
CN201720831932 provides a storage and transportation device for toluene bromide, including an inner tank, a plastic film, an outer tank, a balance foot pad, a water inlet pipe, a water outlet pipe, a top cover, a pressure gauge, a pressure relief valve, a thermometer, an extension pipe and Ice crystal particles, the plastic film is arranged on the inner wall of the inner tank; the outer tank is arranged at the outer end of the inner tank, and forms a cavity with the inner tank; the balance foot pad is fixedly installed at the bottom end of the outer tank ; The water inlet pipe is arranged on one side of the lower end of the outer tank; the water outlet pipe is arranged on the other side of the upper end of the outer tank; the top cover is fixedly installed at the top center of the outer tank; the pressure gauge extends through the top cover to Installed in the extension pipe in the inner tank; the pressure relief valve is fixedly installed on one side of the upper end of the outer tank and connected in the inner tank; the thermometer is fixedly installed on the other side of the upper end of the outer tank and connected in the inner tank; The ice crystal particles are arranged in the cavity between the outer tank and the inner tank. Compared with the existing technology, the beneficial effect of the present utility model is that the new type of p-bromotoluene storage and transportation device has the advantages of simple structure, safe storage and transportation, low manufacturing cost, capable of large quantities of storage and transportation, simple use, etc., and is suitable for popularization and use. .
Figure 1 is a rendering of the overall structure of the utility model; in the figure: 1-inner tank, 2-plastic film, 3-outer tank, 4-balance foot pad, 5-water inlet pipe, 6-water outlet pipe, 7- Top cover, 8-pressure gauge, 9-pressure relief valve, 10-thermometer, 11-extension tube, 12-ice crystal.
Apply [3]
1. Used to prepare 4-bromobenzoic acid
4-Bromobenzoic acid, also known as p-bromobenzoic acid, is white or light pink crystal. 4-Bromobenzoic acid is an important fine chemical that can be used as a raw material for spices and as a neutralizer for 4-bromobenzoate esters.Intermediates, standards for measuring strontium, organic trace analysis for measuring carbon, hydrogen and bromine. There are currently two main methods for producing 4-bromobenzoic acid. One is to oxidize p-bromotoluene with potassium permanganate to produce p-bromobenzoic acid. The cost is too high and the yield is only 80%; the other method is to produce p-bromobenzoic acid. Bromobenzene reacts with acetyl chloride to generate 4-bromoacetophenone, and then 4-bromoacetophenone reacts with sodium hypochlorite to generate 4-bromobenzoic acid. This method has many reaction steps and serious pollution. In view of the problems existing in the above-mentioned existing technologies, it is urgent to research and develop a new process for preparing 4-bromobenzoic acid with less pollution, low cost and high yield.
In view of the problems existing in the existing technology, the present invention CN201810447513.5 provides a preparation method of 4-bromobenzoic acid, which uses oxygen to oxidize p-bromotoluene to prepare 4-bromobenzoic acid. The yield can reach 98%, and the product The purity can reach over 99%, with little pollution and low cost. In order to achieve the above objects of the present invention, the present invention adopts the following technical solution: a preparation method of 4-bromobenzoic acid, which uses p-bromotoluene as the starting raw material, glacial acetic acid as the solvent, oxygen as the oxidant, and utilizes liquid phase oxidation method Catalytically oxidize p-bromotoluene under the action of a catalyst, control the reaction temperature to 75-85°C, end the reaction when the content of p-bromotoluene in the reaction system is less than 0.5wt% of the initial content, and then cool and filter to obtain 4-bromobenzoic acid. crude product and filtrate, and then further purify the crude 4-bromobenzoic acid to obtain a finished product. The melting point of the finished 4-bromobenzoic acid is 252-254°C and the purity is more than 99%.
2. Used to prepare 2-cyano-4’-methylbiphenyl
2-Cyano-4′-methylbiphenyl (trade name: Sartanbiphenyl) is a Sartan antihypertensive drug (such as Losartan, Valsartan, Iprosartan, Iprosartan). Besartan, etc.) key intermediates. Sartan drugs have the characteristics of wide range of effects, significant antihypertensive effect, convenient taking, and few side effects. With a large number of drugs entering the market in recent years, the market usage of 2-cyano-4′-methylbiphenyl intermediates is increasing day by day. , it is particularly important to study the manufacturing technology of 2-cyano-4′-methylbiphenyl.
CN201210505581.5 reports a synthesis method of 2-cyano-4’-methylbiphenyl, which includes the following steps:
1) Dissolve o-bromobenzonitrile into an organic solvent;
2) Add the catalyst, heat to 30~60°C with stirring, and continue stirring for 5~10 minutes;
3) Drop in p-bromotoluene and stir at 30-60°C for 2-4 hours;
4) Cool to room temperature, add dilute hydrochloric acid to adjust pH=6~7, and separate the organic layer
, 5) Extract the aqueous layer with n-hexane, combine the organic layers, wash, dry, distill and recrystallize to obtain light yellow needle-shaped crystals, which is the product 2-cyano-4’-methylbiphenyl.
Main reference materials
[1] CN201310495723.9 A kind of synthesis process of p-bromotoluene
[2] CN201720831932.X A storage and transportation device for p-bromotoluene
[3] CN201810447513.5 Preparation method of 4-bromobenzoic acid
[4] CN201210505581.5 Synthesis method of 2-cyano-4’-methylbiphenyl



 微信扫一扫打赏
微信扫一扫打赏
