[Background and Overview][1]
Resorcinol, also known as resorcinol, has similar properties to phenol. It is a water-soluble colorless crystal at room temperature. It is flammable and has a sweet taste. It is easily soluble in water, alcohol and ether, and is insoluble in chloroform and tetrachloride. It contains carbon and benzene and has a faint special smell. Resorcinol is weakly acidic, but its acidity is stronger than carbonic acid. It will easily gradually turn light red when exposed to sunlight and air for a long time.
Resorcin is an important fine organic chemical raw material, widely used in agriculture, dyes, coatings, medicine, plastics, rubber, electronic chemicals, etc. field. Most of the finished resorcinol products are used to synthesize resins and adhesives. Adhesives such as resorcinol-formaldehyde resin and resorcinol-aldehyde-vinyl adhesion resin do not strengthen acid or strong alkali catalysts under neutral conditions and can be cured at room temperature. They are not affected by water. It does not corrode and does not cause decomposition of the adherend. It can be widely used in the tire industry to bond textile materials and rubber. At the same time, they can also bond textile fibers, wood, light metals, ceramics, plastics and elastomers, etc., so they also have special and important uses in the manufacture of laminated wood, ship hull structures, roof structures and laminated paper. Because the polycondensation reaction and curing speed of resorcin and formaldehyde are very fast, resorcin is often used to modify phenolic resins and urea resins. Adding a small amount of resorcinol to the mold of the above resin can accelerate the curing of the resin and make the product more water-resistant.
Resorcinol is an important dye intermediate, from which a series of azo dyes and fluorescent dyes are synthesized. Resorcinol is used as a fur dye and hair dye when mixed with catechol, amines and oxidants. Resorcinol is used as a developer in the photosensitive industry. Resorcinol can be used as an intermediate to synthesize m-aminophenol, p-aminosalicylic acid, resorcinol benzoate, resorcinol salicylate, resorcinol ethers, etc. Among them, mid-aminophenol is the starting material of anti-tuberculosis drugs. Resorcinol has a certain bactericidal effect, so it is often used to make ointments and apply externally to treat fungal infections. Generally speaking, resorcin derivatives are less toxic than resorcin itself, so resorcin-acetate or n-hexylresorcin are often used as intestinal disinfectants. In addition, because resorcinol and its derivatives can form colored precipitates or solutions with many substances, they can be used as chemical analysis reagents.
[Shape]
This product is white or off-white needle-shaped crystals or powder; it has a slight odor; it slowly turns pink when exposed to sunlight or air. This product is easily soluble in water or ethanol, easily soluble in ether or glycerol, and slightly soluble in chloroform.
Melting point: The melting point of this product is 109~111℃.
[Indications][2]
This product has keratin promoting and keratolytic effects, and has antifungal and antibacterial properties. It is often used for eczema, psoriasis, seborrheic dermatitis, acne, tinea cruris and other diseases. High concentrations are also commonly used for corns, calluses, common warts, etc.
[Pharmacological effects][3]
0.25% to 1% of this product is a cuticle promoter, 5% is a cuticle loosening agent, 20% to 40% is an exfoliating agent, and more than 40% is a corrosive agent. This product has the effect of killing bacteria and fungi. The intensity of the effect is 1/3 of phenol. It also has anti-itching effect and is less irritating to the skin. Easily soluble in water, alcohol and other solvents. It has the effect of inhibiting bacteria and fungi. Different concentrations have effects on the skin. Low concentration (0.5% ~ 1%) can relieve itching and promote keratin formation, and concentration of 5% can release keratin. High concentration (20% ~ 40%) has exfoliating effect. Concentrations above 40% are corrosive.
[Preparations and Specifications] Resorcinol lotion: 3%; Resorcinol ointment: 2% to 20%.
【Usage and Dosage】
0.25% to 5% aqueous solution is used as a wet compress for the exudative and erosive stage of acute eczema and dermatitis; 1% to 10% ointment, lotion or compound liniment is used to treat eczema, ringworm, psoriasis, Acne and seborrheic dermatitis, etc.; 2% to 5% solution can be used as a wound and urethral irrigant.
[Adverse reactions][4]
Can cause contact dermatitis. Because this product can be absorbed through the skin or ulcers, it should not be used in high concentrations or on large areas in infants and young children. Symptoms of poisoning include diarrhea, nausea, vomiting, stomach pain, dizziness, severe or persistent headache, fatigue or weakness, irritability or irritability. , drowsiness, night sweats, bradycardia, and shortness of breath; children may develop methemoglobinemia when this product is applied to wounds. Avoid contact with eyes. This product can dye light-colored hair black and cause skin redness and scaling within a few days after use. Sharing this product with soaps, detergents, acne preparations, preparations containing alcohol or retinoic acid may cause excessive irritation or dryness of the skin.
[Notes][5]
Some patients may experience local redness, stinging, dry skin, etc. after applying the medicine, which usually subsides in 1-2 minutes. Add vitamin C (0.5%-1%) to the solution of this product to prevent oxidation and discoloration. This product is less irritating and can be absorbed through wounds and damaged skin, causing myxedema. This product is forbidden to be mixed with ethyl nitrite. , iron salts, interactions with this product and antipyrine, camphor, chloral hydrate, menthol, phenol: pyrogallol, etc., will liquefy or soften. This crystal will liquefy or soften when it encounters chloral hydrate, chloral hydrate, and iron. B, etc., generate coloredcompound. Silver, mercury, and copper salts can be reduced by this product. [Preparation and specifications] Resorcinol: 0.25%-1% keratolytic agent; 5% keratolytic agent; 20%-40% exfoliating agent; More than 40% corrosive; 0.25%-5Q6 aqueous solution; 1%-10% ointment or lotion.
【Preparation】【6】
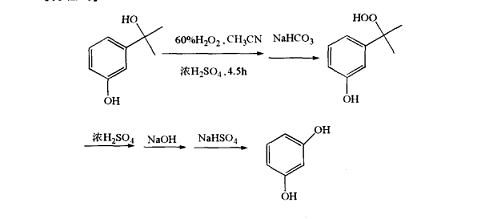
(1) Example 1: Preparation of 3-(2-hydroperoxide-2-propyl)phenol
Add 1.1 g of 60% (weight) hydrogen peroxide aqueous solution and 5 mg of concentrated sulfuric acid to 20 mL of acetonitrile solution dissolved in 3-(2-hydroxy-2-propyl)phenol, and react at 25 ℃ for 4.5 hours. After the reaction is completed, neutralize the reaction mixture with excess (1 g) sodium bicarbonate, filter, and concentrate the filtrate under reduced pressure. Add 50 mL of diethyl ether to the concentrate, and add the ether dispersion with the product dissolved in the water. The reaction product is retained in the ether phase, while the by-product is transferred to water, the ether is distilled off under reduced pressure, and the ether extract is concentrated. The remaining solid product was recrystallized in chloroform to obtain 1.2 g of colorless crystals with a purity of 98.4% (based on DSC) and a yield of 77%. Dissolve 0.5 g of 3-(2-hydroperoxide-2-propyl)phenol in 10 mL of acetonitrile at room temperature, and add 0.59 1% sulfuric acid aqueous solution to it. The solution was left at room temperature for 1 hour, and the retention value of the oxychloride in the solution was measured to be 98% using liquid chromatography.
(2) Example 2: Preparation of resorcinol
Dissolve 16.8 g of 3-(2-hydroperoxide-2-propyl)phenol in 20 mL of toluene, add 5 mg of concentrated sulfuric acid, and heat to reflux for 15 min. After the reaction was completed, the reaction mixture was neutralized, and liquid chromatography analysis revealed that the reaction mixture contained benzene. Sodium bisulfite was added to the reaction mixture to decompose the peroxide, the precipitate was filtered off, and the solvent was evaporated under reduced pressure. The distillation residue obtained 0.77 g of quinone, with a yield of 70%.
(3) Example 3: 3-(2-hydroperoxide-2-propyl)phenol
2.8 g of 60% (weight) hydrogen peroxide aqueous solution (50 mm01) and 0.2 g of sulfuric acid were added to 20 mL of acetonitrile in which 1.34 g (10 mm01) of m-isopropenylphenol was dissolved. solution, the reaction was carried out at 40°C for 30 min. After the reaction, liquid chromatography was used to analyze the reaction mixture containing 0.50 g (3 mm01) of 3-(2-hydroperoxide-2-propyl)phenol.
(4) Example 4: Preparation of 3-(2-hydroperoxide-2-propyl)phenol
2.8 g 60% (weight) hydrogen peroxide aqueous solution (50 mmo1) and 1.25 g phosphoric acid are added to 20 mL of 1.34 g (10 mmo]) m-isopropenylphenol dissolved of acetonitrile solution, react at 50 . C for 24 hours. After the reaction, the reaction mixture was analyzed by liquid chromatography and found to contain 0.59 g (3.5 mmol) of 3-(2-hydroperoxide-2-propyl)phenol.
(5) Example 5: Preparation of 3-(2-hydroperoxide-2-propyl)phenol
2.8 g of 60% (weight) hydrogen peroxide aqueous solution (50 mmo|) and 1.259% phosphoric acid were added to 20 mL of 13.4 g (100 mmo1) of isopropenylphenol. In acetonitrile solution, the reaction was carried out at 50°C for 1 h. After the reaction, liquid chromatography analysis was performed and it was found that the reaction mixture contained 1.68 g (10 mmol) of 3-(2-hydroperoxide-2-propyl)phenol.
[References]
[1] Tian Xuan. Research on low-pollution synthesis process of resorcinol[D]. Nanjing University of Science and Technology, 2007.
[2] Wei Taixing, editor-in-chief Wei Jinghan, Doctor’s Manual of Drugs, Henan Science and Technology Press, 2015.01, page 1562
[3] Editor-in-chief Mao Weian; deputy editors such as Zhang Jian, Wu Jianhua, Liu Jiyong, etc.; editors such as Mao Jingyi, Wang Li, Zhang Lichao, etc., Dermatology Drug Quick Reference Manual, Golden Shield Publishing House, 2014.04, page 29
[4] Shi Haibo, editor-in-chief Wang Kelin, Latest Clinical Drug Manual, Military Medical Science Press, 2008.1, page 1001
[5] Chief editor Li Xinfang Cui Dongbin Chief editor Li Xinfang, Dermatology Drug Quick Reference Manual, Hebei Science and Technology Press, June 2005, 1st edition, page 237
[6] Wen Huiliang, editor-in-chief Liu Chongbo, World New Drug Patent Production Technology, Jiangxi Science and Technology Press, 2007.3, page 330



 微信扫一扫打赏
微信扫一扫打赏
