[Background and Overview][1][2]
2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene, CAS number is 898566-17-1, molecular formula is C18H14FIS, molecular weight 408.27200; PSA is 28.24000; LogP is 6.05800, density 1.533, boiling point 450.2ºC at 760 mmHg, flash point 226.1ºC, refractive index 1.643. If inhaled, move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if eye contact occurs, separate the eyelids and rinse with fluid Rinse with water or saline and seek medical attention immediately. If ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately. Advice to protect rescuers is as follows: Move the patient to a safe place, consult a doctor, and if conditions permit, please show this chemical safety data sheet to the doctor who comes to the scene. If there is a small leak, collect the leaked liquid in a sealable container as much as possible, absorb it with sand, activated carbon or other inert materials, and transfer it to a safe place. Do not flush it into the sewer; if there is a large leak, build a dike or dig a pit. Contain, seal the drainage pipe, cover it with foam to inhibit evaporation, use an explosion-proof pump to transfer it to a tanker or a special collector, and recycle or transport it to a waste treatment site for disposal.
【Preparation】[1][2]
Method 1: Using 2-(4-fluorophenyl)thiophene as raw material, prepare 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene , the reaction equation is as follows:
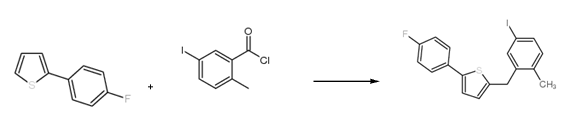
The specific reaction steps for preparing 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene are as follows:
Step 1: 4-Fluoro-phenylmagnesium bromide
Add 2-Me-THF (80ml, 0.1L/mole) to Mg (19.44g, 0.8mol) and stir slowly. Dissolve 1-bromo-4-fluoro-benzene (142.8g, 0.816mole) in 2-Me-THF (200ml, 0.25L/mole) and add 25ml of this solution to the Mg mixture. The resulting mixture was heated to about 43°C and the remaining 1-bromo-4-fluorobenzene solution was added over about 40 minutes while maintaining the mixture at reflux temperature. Rinse the addition funnel to which 1-bromo-4-fluorobenzene was added with 2-methyl-THF (40 mL), and add the cleaning solution to the reaction solution. The resulting mixture was stirred at 90°C for 1 hour and cooled to 20°C to obtain a brown-green solution containing 4-fluoro-phenylmagnesium bromide.
Step 2: 2-(4-fluorophenyl)thiophene
Dissolve 2-bromothiophene (130.4g, 0.8mol) in 2-Me-THF (240ml, 0.3L/mole), and cool the resulting mixture to 2°C, add NiCl 2 (dppe) (2.11g, 4.0mmol), at ≤30°C, add the 4-fluoro-phenylmagnesium bromide solution prepared in step A to obtain a dark red solution. The solution was then stirred at 22°C for 1.5 hours, a solution of acetic acid (91.7ml, 1.6mol) in water (240ml, 0.3L/mol) was added, the resulting mixture was stirred vigorously for 15 minutes, the layers were separated, and water (80ml, 0.1L/mol) was added. mol), wash the organic layer and concentrate in vacuum at 75°C to obtain 2-(4-fluorophenyl)thiophene as brown oil.
Step 3: 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene
Add DCM (350ml, 1L/mol) to 91.7g 5-iodo-2-methylbenzoic acid (91.7g, 0.35mol), stir at 22°C, and add sulfite to the resulting mixture using an addition funnel. Acid chloride (42.5g, 0.35mol), slowly heat the resulting mixture to reflux temperature (at this time the mixture becomes a colorless solution and gas evolution is observed), stir for 1 hour, and cool to 2°C. Aluminum chloride particles (56.0g, 0.42mol) were added to the resulting mixture, stirred at 2°C for 15 minutes, and then 2-(4-fluorobenzene) dissolved in DCM (0.5L/mol) was added using an addition funnel. base) thiophene (0.35 mol, 89.7% w/w) and the temperature was increased to 20 °C. The resulting mixture was stirred at 20°C for 2 hours, then cooled to 2°C, remaining aluminum chloride particles (107.3g, 0.805mol) were added, and the resulting mixture was stirred for 15 minutes. Use a dropping funnel to add acetonitrile (210ml, 0.6L/mol) within 20 minutes at T≤20°C, and add tetramethyldisiloxane (131.6g, 0.98mol) within 5 minutes, and then slowly add the resulting mixture Heat to reflux temperature (42°C), maintain reflux for 3 hours, allow to cool to 22°C, and then stir for 16 hours. Add water (420ml, 1.2L/mol) within 30 minutes at T≤35°C, and stir the resulting mixture vigorously for 15 minutes, separate the layers, wash the organic layer with water (70ml, 0.2L/mol), and then incubate at 50°C Concentrate in vacuo to obtain crude 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene.
Dissolve crude 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene (62.0g, 0.1mol) in ethyl acetate (40ml) and To a mixture of 2-propanol (50 ml), activated carbon (1.2 g) was added to the resulting mixture, and the resulting mixture was heated to reflux and then stirred under reflux for 15 minutes. The resulting mixture was filtered with filter aid, and the filter was washed with ethyl acetate (10 ml). The combined filtrate and washing liquid were cooled to 2°C over 16 hours to cause spontaneous crystallization. The precipitate was filtered, washed with 2-propanol (50 ml), and dried under vacuum at 60°C to obtain solid 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl] Thiophene.
Method 2: Using 5-bromo-2-methylbenzoic acid as raw material to prepare 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene, The specific steps are as follows:
Step 1: At 20°C, put 5-bromo-2-methylbenzoic acid (22.5g, 0.10mol) into a 250mL three-neck round-bottom flask.��Dichloromethane (100mL) and DMF (0.25mL). Oxalyl chloride (12 mL, 0.13 mol) was added to keep the internal temperature below 25°C. Violent gas evolution was observed. The reaction mixture was stirred under argon overnight. The volatiles were removed under reduced pressure, and the resulting residue (acyl chloride compound) was Dissolve in DCM (50 mL) and set aside under nitrogen.
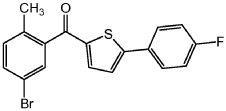
Step 2: Add AlCl 3 (15.0g, 0.11mol) and 100mL methylene chloride into a separate 500mL three-neck round-bottom flask. Cool the suspension to -10°C in an ice bath, then add 2-(4-fluorophenyl)thiophene (18.2g, 0.10mol), followed by the mixture prepared in step 1 above, and after 30 minutes, remove the ice Bath and stir the resulting mixture at room temperature for 2-3 hours. Cool the resulting mixture to -120°C. Slowly add water (20 mL), 2N HCl (20 mL) and heptane (100 mL) to quench to form a precipitate. Stir the resulting mixture for 1 -2h, and then filtered to obtain a yellow solid compound with the following structure:
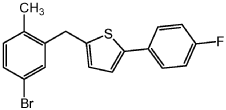
Step 3: Add the compound prepared in step 2 (119g, 0.317mol), triethylsilane (148mL, 0.926 mol), dichloromethane (700 mL) and acetonitrile ( 700 mL). The resulting mixture was cooled to -80°C in an ice bath with stirring, and then trifluoroethyl ether (115 mL, 0.915 mol) was added dropwise, with the temperature not exceeding 0°C. The resulting mixture was warmed to room temperature and stirred overnight, concentrated under reduced pressure, diluted with IPA (1.0 L), filtered and washed with water to obtain a solid. Recrystallize the solid from IPA to obtain a yellow solid:
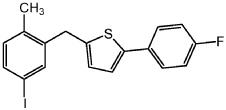
Step 4: Add the compound prepared in step 3 (100 g, 276.80 mmol), sodium iodide (82 g, 553.59 mmol) and copper iodide (2.6 g, 13.84 mmol) into a 1.0L four-neck reaction flask, argon Treat with toluene (261 mL), diglyme (56 mL) and N,N’-dimethyl-ethane-1,2-diamine (2.7 mL, 27.68 mmol) under gas, and warm the resulting mixture to 110°C. After the reaction is complete, the resulting mixture is cooled to room temperature, then filtered through Celite, washed with EtOAc, and extracted with NH4OH. The organic phase is dried (Na2SO4), filtered and concentrated to obtain a solid. Filter the solid and recrystallize with heptane to obtain off-white solid 2-(4-fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene:
[Application][1][2]
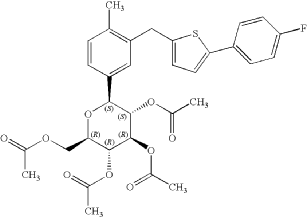
2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene can be used as a pharmaceutical intermediate for the synthesis of other compounds with certain activity, if available To prepare compounds with anti-sodium-dependent glucose transporter (SGLT) inhibitory activity, the structure is as follows:
[Main reference materials]
[1] Walter Ferdinand Maria Filliers;Janssen Pharmaceutica N.V.;Rudy Laurent;Maria Broeckx;Misubishi Tanabe Pharma Corporation;Patrick Hubert J. Nieste;Masanori Hatsuda;Masahiko Yoshinaga;Mitsuhiro Yada;Christopher Teleha .Process for the preparation of compounds useful as inhibitors of SGLT.US2010/99883A1, ;Page/Page column 40-41 ; US 20100099883 A1
[2] ABDEL-MAGID, Ahmed F.; (US).CHISHOLM, Maureen; (US).MEHRMAN, Steven; (US).SCOTT, Lorraine; (US).WELLS, Kenneth M.; (US) ).ZHANG-PLASKET, Fan; (US).NOMURA, Sumihiro; (JP).HONGU, Mitsuya; (JP).KOGA, Yuichi; (JP). PROCESS FOR THE PREPARATION OF COMPOUNDS USEFUL AS INHIBITORS OF SGLT .WO2009/ 35969 A1, ; Page/Page column 77 ; WO 2009/035969 A1



 微信扫一扫打赏
微信扫一扫打赏
