Background and overview[1]
O-trifluoromethylstyrene is a commonly used monomer in the synthesis of polymer materials fluorinated polystyrene. So far, there are two methods for the synthesis of o-trifluoromethylstyrene reported in the literature. The first method is to use trifluoromethylphenylethyl alcohol as the raw material and potassium hydrogen sulfate as the dehydrating agent to perform a heating dehydration reaction. The second method is to use trifluoromethylphenylethyl alcohol as the raw material, use phosphorus pentoxide as the dehydrating agent in anhydrous benzene solvent to perform a heating dehydration reaction. Since o-trifluoromethylstyrene is unstable, current reporting methods are all in acidic conditions. When heated and dehydrated, self-polymerization of o-trifluoromethylstyrene will occur, resulting in a decrease in yield. Therefore, the synthesis of this compound has not yet yielded satisfactory results so far.
Preparation[1-2]
Report 1,
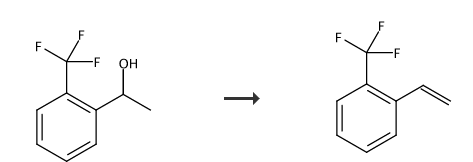
Add potassium dihydrogen phosphate (19g) and 3-trifluoromethylphenylethyl alcohol (190g) into the reaction bottle. Then heat to 100°C and stir for 12 hours. After distillation under reduced pressure, o-trifluoromethylstyrene (65-66°C/41mmHg) was obtained as a colorless liquid with a yield of 90% and a purity of 99.0% (GC).
Report 2,
Add KOSiMe3 (896 mg, 7 mmol, 3.5 equivalents) and tripalladium dibenzylideneacetone (58 mg, 0.1 mmol, 0.05 equivalents) into a 10 mL round-bottomed flask equipped with a magnetic stirring bar. and triphenylphosphine oxide (28 mg, 0.1 mmol, 0.05 equivalent), filled with argon, and then filled the flask with THF (4 mL), 2-bromotrifluorotoluene (450 mg, 2 mmol) and DVDS (500 μL, 2.2) via a syringe. mmol, 1.1 equivalent). The mixture was placed in a preheated oil bath and heated to reflux for 3 hours. After the completion of the reaction was confirmed by GC analysis, the mixture was cooled to room temperature, then filtered through a small silica gel pad (5g), rinsed the silica plug with 50mL ether, and the organic layer was concentrated in vacuo and passed through column chromatography (silica gel, 30mmx140mm, pentane alkane; then RPC-18 silica gel, methanol/water 9:1) purified the crude product to obtain 172 mg (52%) o-trifluoromethylstyrene as a clear colorless oil, yield 172 mg (52%). 1HNMR: (500MHz, CDCl3) 7.66 (d, J=7.8, 1H, HC (7)), 7.63 (d, J=7.8, 1H, HC (4)), 7.52 (t, J=7.6, 1H, (HC (6))), 7.36 (t, J=7.7, 1H, HC (5)), 7.10 (ddd, J=17.3, 11.0 and 2.5, 1H, HC (2)), 5.75 (d, J=17.1, 1H, HtransC (1)), 5.42 (dd, J=11.0 and 1.2, 1H, HcisC (1). 13CNMR : (125MHz, CDCl3) 137.0 (C (3)), 133.3 (C (4)), 132.1 (C (2)), 127.7 (C (5)), 127.3 (C ( 6)), 125.8 (q, C (9)), 125.6 (C (8)), 123.4 (C (7)), 118.2 (C (1)). IR: 3083 (w), 2886 ( m), 2801 (m), 1876 (w), 1611 (s), 1555 (w), 1522 (s), 1480 (m), 1444 (m), 1407 (w), 1351 (s), 1228 ( m), 1187 (s), 1166 (s), 1129 (w), 1061 (m), 990 (m), 946 (m), 887 (m), 821 (s), 751 (m), 691 ( w).
References
[1]CN101781161-A preparation method of o-trifluoromethylstyrene compounds
[2] Denmark S E , Butler C R . Vinylation of Aromatic Halides Using Inexpensive Organosilicon Reagents. Illustration of Design of Experiment Protocols[J]. Journal of the American Chemical Society, 2008, 130(11):p.3690-3704 .



 微信扫一扫打赏
微信扫一扫打赏
