Background and overview[1][2]
The chemical formula of nickel oxalate is NiC2O4·2H2O. Slightly soluble in water and oxalic acid solution, easily soluble in sodium oxalate solution or ammonia water, and soluble in strong acid. Dehydration at 150℃. When heated to below 320°C in a vacuum, it begins to decompose into nickel and carbon dioxide. It is obtained by drying the precipitate generated by adding oxalic acid solution while heating nickel (II) hydroxide or nickel (II) salt, or adding a calculated amount of potassium oxalate to nickel (II) salt solution. Nickel oxalate can be used as a catalyst to prepare fine powdered nickel.
In addition, nickel oxalate is used to improve the sintering properties of high manganese copper damping alloys. Studies have shown that in the powder metallurgical process of preparing nickel-containing manganese-copper alloys, the main role of using nickel oxalate instead of nickel powder as the donor of nickel element is : ① The fine nickel element produced by the thermal decomposition of nickel oxalate has a low melting point and can fuse elemental manganese powder at a lower temperature, thereby reducing the thermal resistance interface, improving the thermal conductivity and temperature uniformity of the sintered billet, thereby improving the manganese-copper Uniformity of sintered alloy;
② In the temperature range where nickel oxalate decomposes, the surface of the compact has not been sintered and densified, so the water and carbon dioxide gas produced by the decomposition of nickel oxalate are continuously discharged from the surface of the compact, preventing the formation of a dense sealing layer on the surface of the compact, making the surface in The porous state is conducive to the discharge of adsorbed water on the surface of the mixed powder during the low-temperature heating stage; during the high-temperature sintering stage, it is conducive to hydrogen entering the sintered body and reducing a small amount of oxidized metal elements;
③ Nickel oxalate is a fine powder and is not prone to plastic bonding, so it is conducive to uniform mixing of ingredients; ④ The fine high-energy state nickel powder obtained by decomposition is easy to sinter and diffuse with elements such as manganese, copper, iron, and aluminum, thereby promoting Liquid phase formation and sintering process. Therefore, nickel oxalate has broad application prospects.
Structure
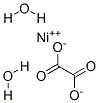
Apply[3]
Nickel oxalate can be used to prepare metal nickel powder and can also be used as a catalyst. Examples of its application are as follows:
Used to prepare a high-purity nickel powder with high properties and stable quality. This is achieved through the following technical solution: first dissolve the pretreated nickel plate in nitric acid, filter to remove impurities and then precipitate with oxalic acid solution, then wash and dry the precipitate, then pyrolyze it in a hydrogen reducing atmosphere, and finally crush the reduction product , sieved to obtain high-purity nickel powder. Specifically, it includes the following steps:
1. Dissolve the nickel plate in the nitric acid aqueous solution to make a nickel nitrate aqueous solution;
2. Filter the nickel nitrate aqueous solution to remove impurities and then add the oxalic acid aqueous solution to react to obtain nickel oxalate precipitate;
3. Wash the nickel oxalate precipitate, dry the nickel oxalate precipitate and place it in a reduction furnace. Control the temperature to 420-430°C and perform pyrolysis and reduction under the control of hydrogen atmosphere for 110-130 minutes, and then pyrolyze the obtained The reduction product is crushed with a stirring ball mill, and the nickel powder with a particle size of 250 mesh + 325 mesh or more is screened out as primary reduced nickel powder;
4. Place the primary reduced nickel powder in the reduction furnace, control the temperature to 520-530℃, perform pyrolysis and reduction under the control of hydrogen atmosphere for 110-130 minutes, and crush the obtained pyrolysis reduction product with a stirring ball mill. The particle size screened out is 250 mesh +325 mesh (250 mesh means that it can pass through the 250 mesh sieve, +325 mesh means that it cannot pass through the 325 mesh sieve, 250 mesh +325 or 250 mesh +325 means it can pass through The product is made from nickel powder that passes through 250 mesh sieve but cannot pass through 325 mesh sieve).
The obtained nickel powder has high purity and stable quality, and can meet the usage requirements of klystrons and radar thyratrons for nickel powder; optimized temperature control and crushing conditions are used in the process to make the nickel powder particles uniform and with high bulk density. ; The production equipment is simple and easy to operate.
Preparation[4][5]
Method 1: A method for preparing spherical nickel oxalate, involving a hydrometallurgical method for producing spherical nickel oxalate.
It is characterized in that its preparation process uses nickel salt solution and ammonium oxalate solution as raw materials. First, liquid ammonia is used to adjust the pH of the nickel solution, and then ammonium oxalate solution is added to perform a precipitation reaction to obtain nickel oxalate, which is filtered, washed, and dried to prepare Obtain spherical nickel oxalate product.
A method for preparing spherical nickel oxalate of the present invention. The process is to select any nickel salt among nickel sulfate, nickel chloride or nickel nitrate, and adjust the pH of the solution to 7.5~8.5 by passing liquid ammonia. Add ammonium oxalate solution until the nickel content in the supernatant is ≤0.5g/L, which is the end point. Filter, wash, and dry to obtain a spherical nickel oxalate product. The content of nickel oxalate is ≥98.5%, and the microscopic morphology is spherical when visually observed under a 200x microscope.
Method: A method for preparing dendritic nickel oxalate,
Includes the following process steps: prepare a nickel chloride solution with a nickel content of 120-180g/l for use; continuously add ammonia water to the nickel chloride solution until the pH value of the solution is 8.0~9.0; control the solution temperature at 60°C ~70°C, continuously add oxalic acid solution dropwise under stirring until the nickel content in the supernatant is ≤0.5g/L, filter the solution after the reaction, and wash the precipitate. After drying the precipitate, the dendritic nickel oxalate product is obtained. .
The present invention can prepare dendritic nickel oxalate with good morphology, good crystal morphology controllability and simple process; because of its special morphology, the dendritic nickel oxalate prepared by the present invention can be used to make nickel powder. Finally, it has special properties and is widely used in the battery industry. Its product price is also much higher than that of electrolytically reduced nickel powder.
Main reference materials
[1] Compound Dictionary
[2] Luo Fenghua; Wang Guan; Lu Fengshuang; Zhang Jianfu; Zhao Dongliang; Zhang Jiansheng. Method of using nickel oxalate to improve the sintering properties of high manganese copper damping alloy. CN201611135462.X, application date 20161212
[3] Zhang Kang; Tan Chengbo; Chen Zhiliang. Metal nickel powder and its preparation method. CN200910194758.2, application date 20090828
[4] Wang Jinrui. A method for preparing spherical nickel oxalate CN201210000153.7, application date 20120104
[5] Yang Zhiqiang; Li Baoping; 20140430



 微信扫一扫打赏
微信扫一扫打赏
