Diethylene glycol butyl ether acetate is a colorless and transparent liquid with a boiling point of 246°C. There are two groups, ether and ester, in its molecular structure. It has the dual characteristics of fatty ether and carboxylic acid ester and can be mixed with water, alcohol, A mixture of ether, acetone and most oils has good compatibility with fibers, resins, paints, coatings, inks, oil-soluble dyes, etc. It can be used as a solvent for couplers in photosensitive materials.
preparation
Other synthesis methods of diethylene glycol butyl ether acetate mainly include: (1) using acetyl chloride and Diethylene glycol butyl ether is used as the raw material for acyl chlorination. This method has low reaction temperature, slow reaction speed, and high corrosivity; (2) The transesterification method of ethyl acetate and diethylene glycol butyl ether is used. Although the product of this method is recovered The yield is relatively high, but the catalyst used such as sodium methoxide is very dangerous; (3) The direct esterification method uses acetic anhydride and diethylene glycol butyl ether as raw materials. Although this method has a higher product yield, the raw materials used are expensive. Not suitable for industrialization.
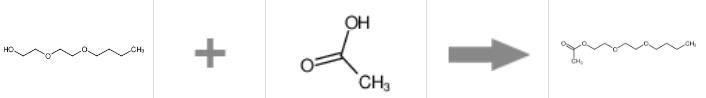
At present, the catalysts used in the direct esterification reaction of acetic acid mainly include AICI3, molecular sieve-5A supported 12-tungstosilicic acid, Although the selectivity and activity of solid acid catalysts such as phosphomolybdenum heteropoly acid and aluminum sulfate immobilized on silica gel are relatively high, the process is complex, the activity is poor after repeated use, and the product quality is unstable.
In a 250 mL four-necked flask, add diethylene glycol butyl ether, a certain amount of acetic acid and strong acidic cation exchange Resin, install thermometer, stirrer, water separator. After filling the water separator with cyclohexane, install the condenser tube and start the stirrer. When the temperature reaches about 84°C, reflux begins and water is generated, and the timer starts. The reaction temperature is controlled at 95-100°C. Heat and reflux until no water is produced. After cooling, move the reaction solution to another four-necked flask. Distill cyclohexane (recovered and reused) and excess acetic acid under normal pressure. Collect under normal pressure distillation at 244-248°C. The colorless and transparent liquid is diethylene glycol butyl ether acetate product.
Conclusion
This article uses strong acidic cation exchange resin to catalyze the synthesis of diethylene glycol butyl ether acetate product. The experimental results show that this method has fast reaction speed and high reaction selectivity. After the reaction is completed, Filtering and removing it can eliminate alkali washing, water washing and other processes, simplifying the production process. Except for a small amount of waste water produced during the esterification reaction, there is basically no discharge of three wastes.
References
[1] US2011/184207 A1, ;



 微信扫一扫打赏
微信扫一扫打赏
