In order to study the polymerization reaction of cellulose components in paper and isocyanate, this paper uses cotton fiber as raw material for polyurethane treatment. It can be seen from the table below that with the increase of the amount of TDI, there are more TDI and its polymers between the fibers to play a bridging role, and the tightness of the paper continues to increase, while reflecting the decrease in air permeability.
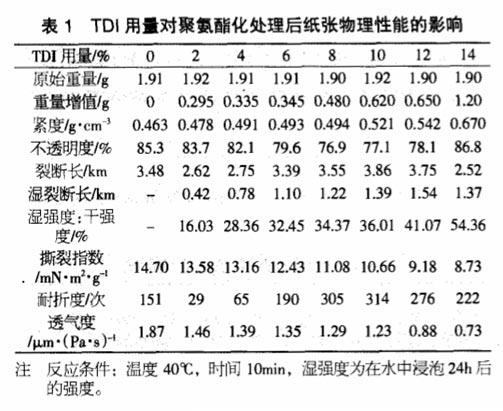
When the amount of TDI is less than 4%, the breaking length and folding strength of the paper are all decreased compared with the base paper. itself hydrogen bonding.
When the TDI content increased from 6% to 10%, the breaking length and folding resistance of the paper gradually increased, indicating that the paper was polyurethaneized through the hydroxyl reaction between the diisocyanate and the fiber, so that the polyurethane structure was in the fiber. Bridge between them, convert hydrogen bonding into chemical bonding, and improve the strength of the paper, especially the wet strength.

When the addition amount is more than 10%, the tensile index and folding endurance show a downward trend again, which is due to the increase of the brittleness of the paper due to the excessive amount of TDI. With the increase of TDI content, the improvement of wet strength is very significant. This kind of processed paper is expected to be used in bag paper, packaging paper, map paper and other industrial papers that require high wet strength.
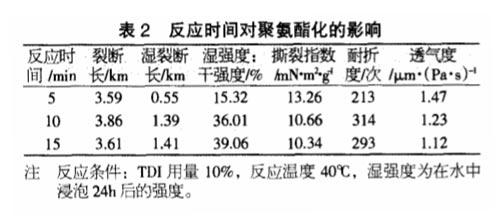
Effect of Reaction Time on Polyurethane
As the reaction time increases, TDI can react more fully with the hydroxyl groups in the paper, so when the reaction time increases from 5 minutes to 10 minutes, the breaking length and folding endurance after polyurethaneization both increase, indicating that The paper is denser; when the reaction continues, the fracture length and folding strength decrease, which may be due to the reaction of the -N=C=O group in TDI and the free -OH in the air, which makes the paper surface generate white matter and reduces the The strength of the paper.
Effect of Reaction Temperature on Polyurethane
From the table below, it can be seen that the strength of TDI paper after polyurethaneization increases gradually with the increase of temperature, but since the melting point of solvent THF is 66°C, when the reaction temperature is close to the melting point of the solvent, THF volatilizes too fast and the reaction is not good. Completely, so the optimum reaction temperature for polyurethaneization is 60°C.
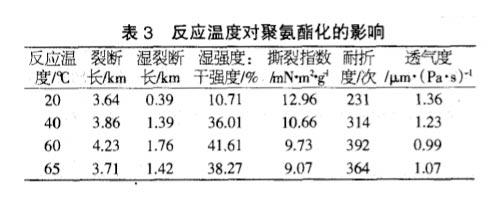
To sum up, when the amount of TDI is 10%, the reaction time is 10 minutes, and the reaction temperature is 60°C, the breaking length of the paper after polyurethaneization is increased by 21.55%, and the wet strength is 41.61% of the dry strength, far exceeding the general wet strength. According to the requirement of strong paper (the value of wet strength/dry strength is more than 15%), the folding endurance is increased by 1.6 times, and the air permeability is reduced to 52.94% of the base paper.



 微信扫一扫打赏
微信扫一扫打赏
