Polycarbonate (PC) is a highly transparent and impact-resistant engineering plastic used in the manufacture of eyeglass lenses, camera lenses, DVDs, automotive headlights and bulletproof glass.
The industrial production of polycarbonate is mainly through the interfacial polymerization method, which makes highly reactive phosgene and alcohol react on the interface of water and organic solvent. However, since phosgene is highly toxic, from the viewpoint of safety, research on a synthesis method that does not use phosgene has been actively conducted.
In recent years, phosgene substitutes (diphenyl carbonate, etc.) have been used to synthesize PCs with relatively low molecular weights, but for the manufacture of high-end PCs with larger molecular weights, in addition to using more reactive traditional phosgene , no other method was found.
In order to develop a new chemical reaction capable of synthesizing safe, cheap, simple and low-environmental load carbonic acid compounds, Kobe University Graduate School of Science Akihiko
Associate Professor Tsuda’s research group used the light-controlled organic synthesis method it developed to develop interfacial polymerization reactions.
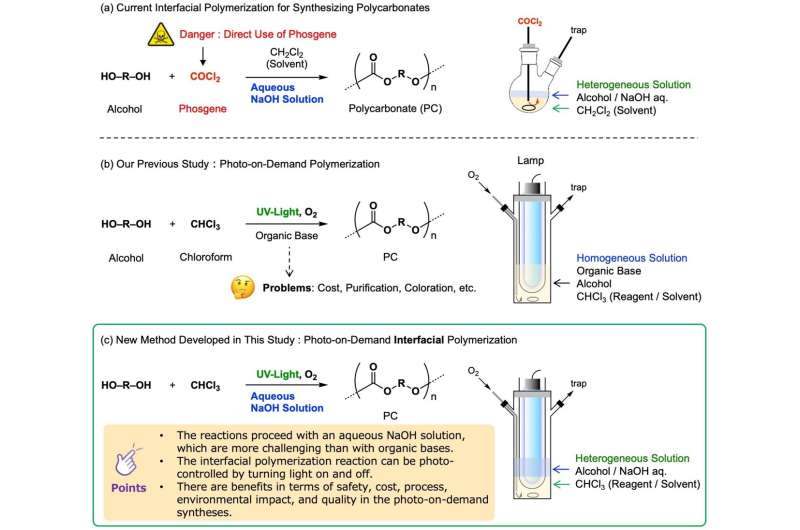
They found that when ultraviolet rays irradiate a mixed solution of sodium hydroxide, chloroform, and ethanol (in a three-phase separation state of gas phase, water phase, and organic phase), and oxygen is introduced into it at the same time, a reaction occurs at the interface, which can produce high yields. Get the target polycarbonate efficiently. This new method is suitable for the small-scale synthesis of various carbonate products.
The research team has obtained patents for the method of synthesizing carbonate compounds at the light-controlled interface in the United States, Singapore, Japan, China, Germany and eight other countries. A paper on the research is published in ACS Omega.
The research team was able to synthesize carbonic acid compounds safely, cheaply, and easily with low environmental load, simply by irradiating a commercially available mixed solution of chloroform, ethanol, and sodium hydroxide with ultraviolet rays. In general, since sodium hydroxide decomposes chloroform and phosgene, although the decomposition was expected to prevent the reaction, it was surprisingly accelerated, contrary to expectations.
Currently, most polycarbonates are made by reacting phosgene with alcohols. In the synthetic method presented here, direct handling of phosgene is not required because the required amount of phosgene is generated by shining light onto the chloroform solvent.
The research team successfully synthesized four commercially available carbonates, three fluorinated carbonates (used as phosgene substitutes), three general-purpose polycarbonates, one specific fluorinated polycarbonate, and six urea derivatives.
Whereas the traditional method of directly using phosgene is suitable for the large-scale production of a few types of carbonates, this new synthetic method is suitable for the small-scale production of many polycarbonates. This contributes to a carbon-neutral sustainable society with low CO2 emissions, low energy consumption and low waste. This study was awarded the “JST
A-STEP SEED Oriented Results” support.



 微信扫一扫打赏
微信扫一扫打赏
