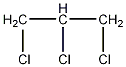
Structural formula
| Business number | 02AM |
|---|---|
| Molecular formula | C3H5Cl3 |
| Molecular weight | 147 |
| label |
Aliphatic halogenated derivatives |
Numbering system
CAS number:96-18-4
MDL number:MFCD00000946
EINECS number:202-486-1
RTECS number:TZ9275000
BRN number:1732068
PubChem number:24869995
Physical property data
1. Properties: colorless to light yellow liquid with chloroform smell. [1]
2. Melting point (℃): -14.7[2]
3. Boiling point (℃): 156.8[3]
4. Relative density (water=1): 1.39 (20℃)[4]
5. Relative vapor density (air = 1): 5.0[5]
6. Saturated vapor pressure (kPa): 1.33 (46℃)[6]
7. Heat of combustion (kJ/mol): -1733.0[7]
8. Critical pressure (MPa): 3.87[8]
9. Octanol/water partition coefficient: 2.27[9]
10. Flash point (℃): 71.1 (CC); 82.2 (OC) [10]
11. Ignition temperature (℃): 304[11]
12. Explosion upper limit (%): 12.6[12]
13. Explosion lower limit (%): 3.2[13]
14. Solubility: Slightly soluble in water, soluble in ethanol, ether, oils, lipids, and paraffin. [14]
15. Viscosity (mPa·s, 20ºC): 0.2505
16. Flash point (ºC, closed): 73.3
17. Flash point (ºC, open): 78.9
18. Vapor pressure (kPa, 9.0ºC): 0.13
19. Vapor pressure (kPa, 46.0ºC ): 1.33
20. Heat of evaporation (KJ/mol, b.p.): 40.56
21. Heat of combustion (KJ/mol, liquid): 1735.9
22. Specific heat capacity (KJ/(kg·K), 20ºC): 1.235
23. Volume expansion coefficient (K-1, 20ºC): 0.00096
24. Relative density (25℃, 4℃): 1.3832
25. Refractive index at room temperature (n25): 1.4812
26. Solubility Parameter (J·cm-3)0.5: 20.148
27.van der Waals area (cm2·mol -1): 8.690×109
28. van der Waals volume (cm3·mol -1): 62.720
29. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -182.9
30 .Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -230.6
31. Liquid phase standard hot melt (J·mol-1·K-1): 172.4
Toxicological data
1. Acute toxicity[15]
LD50: 108μl (150mg)/kg (rat oral); 369mg/kg ( Mouse oral); 372μl (517mg)/kg (rabbit transdermal)
LC50: 3400mg/m3 (mouse inhalation, 2h)
2. Irritation[16]
Rabbit transdermal: 500μl (24h), mild irritation.
Rabbit eye: 100μl, moderate irritation.
3. Mutagenicity [17] Microbial mutagenicity: Salmonella typhimurium 500ng/dish. DNA damage: human lymphocytes 2mmol/L. Cytogenetic analysis: Rats inhaled 800μg/L. Sister chromatid exchange: hamster lung 300 μmol/L.
4. Carcinogenicity[18] IARC Carcinogenicity��Comment: G2A, possible human carcinogen.
Ecological data
1. Ecotoxicity[19]
LC50: 42mg/L (7d) (Rainbow killifish); 109mg/L (48h) (Medaka)
EC50: 45mg/L (24h) (Daphnia)
2. Biodegradability [20]
Aerobic biodegradation (h): 4320~8640
Anaerobic biodegradation (h): 17280~34560
3. Non-biodegradability[21]
Photooxidation half-life in air (h): 61~613
First-order hydrolysis half-life (h ): 44
4. Other harmful effects [22] This substance is harmful to the environment and has an accumulation effect in groundwater.
Molecular structure data
1. Molar refractive index: 30.45
2. Molar volume (cm3/mol): 112.5
3. Isotonic specific volume (90.2K ): 264.2
4. Surface tension (dyne/cm): 30.3
5. Polarizability: 12.07
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 25.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is slightly corrosive to metals. When water is present, it decomposes into highly corrosive hydrogen chloride. Light can accelerate this decomposition. The acidity of 1,2,3-trichloropropane that has been stored or recycled for a long time should be checked before use. Toxic gases are produced during pyrolysis or combustion, so contact with red-hot objects should be avoided.
2. When heated with solid potassium hydroxide, hydrogen chloride is removed, and the main product of the reaction is 1,3-dichloropropene and a small amount of 2,3-dichloropropene. It is heated under pressure with water or sodium bicarbonate aqueous solution in the presence of copper to generate glycerol.
3. Stability[23] Stable
4. Incompatible substances[24] Strong oxidizing agent, strong alkali
5. Conditions to avoid contact[25] Moist air, light, heat
6. Polymerization hazard[26] No polymerization
7. Decomposition products[27] Hydrogen chloride
Storage method
Storage Precautions[28] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. α-Propylene chloride chlorination method: Propylene is chlorinated at high temperature to obtain allyl chloride. After washing and separation, it is then chlorinated at low temperature and fractionated to obtain the finished product.
![]()
2. Dichloroisopropanol Law.
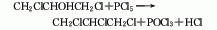
Purpose
1. Used to produce pesticides, organic synthesis, and gas chromatography comparison samples. Used as a paint stripper for varnishes and coatings, and a solvent for engine cleaning. It can also be used as a raw material for pesticides such as chlormequat and Oat Di No. 1.
2. Used as solvent and intermediate. [29]



 微信扫一扫打赏
微信扫一扫打赏
