Structural formula
| Business number | 01K9 |
|---|---|
| Molecular formula | C5H12O |
| Molecular weight | 88.15 |
| label |
tert-amyl alcohol, Dimethylethylmethanol, The third pentanol, Dimethylethyl alcohol, 2-Methyl-2-butanol, dimethyl ethyl carbinol, tert-Pentyl alcohol, tert-Pentanol, 2-Methyl-2-butanol, Dimethyl ethyl carbinol, 1,1-Dimethyl-1-propanol, CH3CH2C(CH3)2OH, solvent for cellulose esters, Solvent for coating paint, varnish solvent, mineral flotation agent, Co-solvent for nitrocellulose spray paint |
Numbering system
CAS number:75-85-4
MDL number:MFCD00004478
EINECS number:200-908-9
RTECS number:SC0175000
BRN number:1361351
PubChem number:24854418
Physical property data
1. Properties: Colorless and transparent liquid with a special pungent odor.
2. Boiling point (ºC, 101.3kPa): 102.0
3. Melting point (ºC): -8.8
4. Relative density (g/mL, 20/4ºC): 0.8090
5. Relative density (25℃, 4℃): 0.8044
6. Refractive index (n20ºC): 1.4050
7 . Viscosity (mPa·s, 25ºC): 3.70
8. Flash point (ºC, opening): 21
9. Heat of evaporation (KJ/kg, b.p.): 444.63
10. Heat of fusion (KJ/kg): 52.75
11. Heat of formation (KJ/mol): -403.73
12. Specific heat capacity (KJ/( kg·K), constant pressure): 3.15
13. Critical temperature (ºC): 270.55
14. Vapor pressure (kPa, 17.2ºC): 1.33
15. Solubility (%, water, 30ºC): 14.0
16. Volume expansion coefficient (K-1): 0.00133
17. Dissolution Property: Slightly soluble in water, can dissolve methanol, ethanol, acetone, ethyl acetate, oleic acid, stearic acid and paraffin, etc.
18. Refractive index at room temperature (n25): 1.4020
19. Critical pressure (MPa): 3.71
20. Eccentricity factor: 0.483
21. Solubility parameter (J·cm-3)0.5: 20.759
22. van der Waals area (cm2·mol-1): 9.170×109
23. van der Waals volume ( cm3·mol-1): 62.610
24. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): 3353.1
25. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -329.7
26. Gas phase standard entropy (J·mol-1·K-1): 362.8
27. Gas phase standard free energy of formation (kJ·mol-1 ): -165.18
28. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3303.06
29 . Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -379.49
30. Liquid phase standard entropy (J·mol-1 sup>·K-1): 229.3
31. Liquid phase standard free energy of formation (kJ·mol-1): -175.14
32. Liquid phase standard hot melt (J·mol-1·K-1): 251.6
Toxicological data
1. Acute toxicity
Human LDLO: 441 mg/kg
Rat oral LD50: 1000mg/kg
Rat abdominal LDL0: 1530mg /kg
Rat subcutaneous LDL0: 1400mg/kg
Rat rectal LDL0: 1400mg/kg
Mouse caliber LD50: 2500mg/kg
Mouse abdominal LDL0: 2mg/kg
Mouse intravenous LDL0: 610mg/kg
Rabbit caliber LD50: 2028mg/kg
Frog rectum LDL0: 4mg/kg
2. It is of low toxicity. Physiological effects are similar to pentanol. Among amyl alcohol isomers, the anesthetic properties decrease in the order of secondary amyl alcohol, tert amyl alcohol, and isoamyl alcohol.
Ecological data
None
Molecular structure data
1. Molar refractive index: 26.71
2. Molar volume (cm3/mol): 108.6
3. Isotonic specific volume (90.2K ): 242.9
4. Surface tension (dyne/cm): 25.0
5. Polarizability (10-24cm3): 10.59
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 39.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Has the chemical reactivity of tertiary alcohols. Among amyl alcohol isomers, the anesthetic properties decrease in the order of secondary amyl alcohol, tert amyl alcohol, and isoamyl alcohol.
Moderately toxic, irritating to eyes, nose and respiratory organs. Inhaling its vapor can cause dizziness, headache, cough, nausea, tinnitus, delirium, and in severe cases, methemoglobinopathy, diabetes, etc. The oral LD50 in rats is 1000mg/kg.
Storage method
This product should be stored sealed in a cool, dark and ventilated place.
Packed in 165kg iron drum. Ventilated, dry, fireproof, explosion-proof, sealed storage. It should be protected from violent impacts, sun and rain.
Synthesis method
1. It is obtained by using acetone and acetylene as raw materials, alkynylation and hydrogenation. Dissolve acetylene in liquid ammonia, mix it with acetone and catalyst and send it to the alkynylation reactor. The reaction solution is flashed to separate unreacted acetylene and ammonia, and the unreacted acetone is evaporated to obtain methyl butynol. It enters the hydrogenation reactor and reacts to generate tert-amyl alcohol, which is dehydrated and refined to obtain the product.
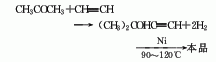
2. Add water from isopentene Become.
3. Produced by fractional distillation of mixed alcohols generated by chlorination and hydrolysis of pentane. The raw material pentane is first dehydrated with anhydrous hydrogen chloride, evaporated and then mixed with chlorine into the reactor. The reaction temperature is 250-300°C. The reaction product is distilled and separated through four distillation towers to obtain pentane chloride. In the hydrolyzer, sodium oleate is used as a catalyst and sodium hydroxide aqueous solution is used to hydrolyze chloropentane. The crude amyl alcohol is separated from water and distilled to obtain pentanol containing 59% primary alcohol, 36% secondary alcohol, and 5% tertiary alcohol. . By using acetone and acetylene as raw materials, a product with a purity of 99% can be obtained. Raw material consumption quota: acetone 1040kg/t, calcium carbide 1500kg/t, hydrogen (99.5%) 650m3, liquid ammonia 135kg/t, potassium carbonate 340kg/t.
Refining method: Use anhydrous potassium carbonate, calcium oxide, calcium hydride or metallic sodium to reflux and then fractionate.
4. Preparation method:
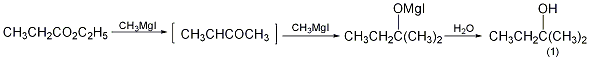
Methyl magnesium iodide prepared by reacting 102g (1.0mol) of ethyl propionate (2) and 49g (2mol) iron filings and 284g (2.0mol) methyl iodide, according to the above 2-methyl magnesium iodide The operating method of -2-pentanol is to collect the fraction between 100 and 102°C to obtain 2-methyl-2-butanol (1). [1]
Purpose
1. Organic synthesis, solvent, and high purity can be used as qualitative and quantitative standard materials in gas chromatography analysis. It is used as a solvent for various cellulose esters, cellulose ethers, coating paints, varnishes, mineral flotation agents, pharmaceutical raw materials and nitrocellulose spray paint co-solvents.
2. Mainly used as raw materials for the new pesticide fentanin, artificial high-grade fragrance musk, seed protection agent triazolol and color film coloring agent; also Can be used for pinacolone, triadimefon, etc.
Qualitative and quantitative reference materials in chromatographic analysis. It is used as a solvent for various cellulose esters, cellulose ethers, coating paints, varnishes, mineral flotation agents, pharmaceutical raw materials and nitrocellulose spray paint co-solvents.
2. Mainly used as raw materials for the new pesticide fentanin, artificial high-grade fragrance musk, seed protection agent triazolol and color film coloring agent; also Can be used for pinacolone, triadimefon, etc.



 微信扫一扫打赏
微信扫一扫打赏
