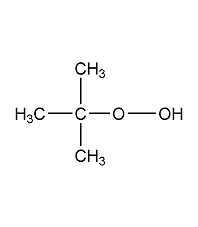
Structural formula
| Business number | 01KD |
|---|---|
| Molecular formula | C4H10O2 |
| Molecular weight | 90.12 |
| label |
1,1-dimethylethyl-hydroperoxide, 3rd butyl hydroperoxide, tert-butyl hydroperoxide, tert-butyl hydroperoxide, tert-butyl peroxide, Terbutanol peroxide, 1,1-Dimethylethyl hydroperoxide, Butylhydroperoxid, 2-Hydroperoxy-2-methylpropane, (CH3)3COOH, oxidizing agent |
Numbering system
CAS number:75-91-2
MDL number:MFCD00002130
EINECS number:200-915-7
RTECS number:EQ4900000
BRN number:1098280
PubChem number:24851948
Physical property data
1. Properties: water white emulsion. General products are solutions of non-volatile solvents. [1]
2. Melting point (℃): -8[2]
3. Boiling point (℃): 35 (2.66kPa)[3]
4. Relative density (water = 1): 0.88[4]
5. Relative vapor density (air = 1): 2.07[5]
6. Saturated vapor pressure (kPa): 2.27 (35~37℃)[6 ]
7. Critical pressure (MPa): 4.34[7]
8. Octanol/water partition coefficient: 0.94[8]
9. Flash point (℃): 26.7[9]
10. Ignition temperature (℃): 238[10]
11. Explosion upper limit (%): 10[11]
12. Explosion lower limit (% ): 5[12]
13. Solubility: Slightly soluble in water, easily soluble in most organic solvents such as ethanol and ether, and sodium hydroxide aqueous solution. [13]
14. Refractive index at room temperature (n20): 1.4010
15. Refractive index at room temperature (n25): 1.3983
16. Solubility parameter (J·cm-3)0.5: 20.822
17.van der Waals area (cm2·mol-1): 8.420×109
18. van der Waals volume (cm3·mol-1): 56.080
19. Gas phase standard heat of combustion (enthalpy) (kJ·mol -1): -2757.3
20. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -245.9
21. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2709.6
22. Liquid phase standard claimed heat (enthalpy) (kJ· mol-1): -293.6
23. Liquid phase standard hot melt (J·mol-1·K-1): 136.6
Toxicological data
1. Acute toxicity[14]
LD50: 370mg/kg (rat oral); 790mg/kg (rat transdermal); 460μl (427.8mg)/kg (rabbit transdermal)
LC50: 1840mg/m3 (rat inhalation, 4h)
2. Irritation[15]
Rabbit transdermal: 500mg (24h), severe stimulation.
Rabbit eye: 100mg (24h), moderate irritation.
Ecological data
1. Ecotoxicity[16] LC50: 220mg/L (48h) (medaka)
2. Ecological degradability[17] In the air, when the concentration of hydroxyl radicals is 5.00×105 /cm3, the degradation half-life is 5d (theoretical).
Molecular structure data
1. Molar refractive index: 23.82
2. Molar volume (cm3/mol): 98.4
3. Isotonic specific volume (90.2K ): 222.7
4. Surface tension (dyne/cm): 26.1
5. Polarizability (10-24cm3): 9.44
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.6
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 35.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Unstable
2. Incompatible substances[19] Reducing agents, strong acids, flammable or combustible substances, active metal powders
3. Conditions to avoid contact[20] Heat, sun exposure, impact
4. Polymerization hazard[21] No polymerization
5. Decomposition products[22] Methane, Acetone, tert-butyl alcohol
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature does not exceed 30°C and the relative humidity does not exceed 80%. Keep container tightly sealed. It should be stored separately from reducing agents, acids, flammable materials and active metal powders, and avoid mixed storage. It should not be stored for a long time to avoid deterioration. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. Vibration, impact and friction are prohibited.
Synthesis method
1. Tert-butyl alcohol reacts with sulfuric acid to form tert-butyl hydrogen sulfate, which is then reacted with hydrogen peroxide. Add tert-butyl alcohol to the reaction pot, add hydrogen peroxide at 35°C with stirring, then raise the temperature to 50°C, add 70% sulfuric acid dropwise, react for 5 hours after the addition, keep the temperature at 55-60°C, let stand and separate, take The upper oil layer was dried with anhydrous sodium sulfate and filtered to obtain tert-butyl hydroperoxide.
2. Preparation method:
In a reaction bottle equipped with a stirrer, thermometer, and dropping funnel, add 74g (1.0mol) of tert-butyl alcohol (2) and keep in an ice bath Cool, add 140g 70% sulfuric acid (1.0 mol), and react at 5°C to generate mono-tert-butyl sulfate. Then add 126g (1.0mol) of 27% hydrogen peroxide dropwise at 0 to 5°C for about 30 minutes. After adding, let it sit at room temperature overnight. Separate the organic layer, neutralize it with a suspension composed of magnesium carbonate and 10mL water, wash with 20mL water, and dry over anhydrous magnesium sulfate to obtain 70.5g of product, which contains 66% tert-butyl hydroperoxide (1), 34% Di-tert-butyl peroxide, active oxygen content 11.74%. Use a packed column for vacuum fractionation. Collect the fraction at 12-13°C/2.66kPa as di-tert-butyl peroxide, and the fraction at 4.5-5°C/266Pa as compound (1). Never distill at normal pressure. [25]
Purpose
1. Catalyst for polymerization reaction. Introduction of peroxide groups into organic molecules. Ethylene monomer polymerization accelerator.
2. Used as catalyst, bleaching powder and deodorant, cross-linking agent for unsaturated polyester, polymerization initiator, and rubber vulcanizing agent. [24]



 微信扫一扫打赏
微信扫一扫打赏
