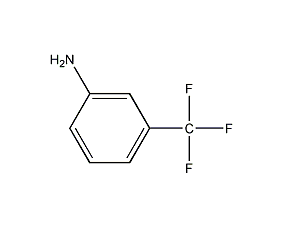
Structural formula
| Business number | 02DH |
|---|---|
| Molecular formula | C7H6F3N |
| Molecular weight | 161 |
| label |
m-aminotrifluorotoluene, m-trifluorotoluidine, m-Trifluoromethylaniline, m-Aminotrifluorotoluidine, α,α,α-Trifluoro-m-toluidine, 3-(Trifluoromethyl)aniline, m-Aminobenzotrifluoride, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:98-16-8
MDL number:MFCD00007797
EINECS number:202-643-4
RTECS number:XU9180000
BRN number:387672
PubChem number:24890833
Physical property data
1. Properties: colorless oily liquid with a smell like aniline.
2. Boiling point (℃): 187.5
3. Relative density (12.5℃): 1.304
4. Refractive index (12.5℃): 1.482~ 1.486
5. Solubility: Insoluble in water, soluble in alcohol and ether. It turns brown when exposed to light oxidation.
Toxicological data
1. Acute toxicity: Rat oral LD50: 480mg/kg; Rat inhalation LC50: 440 mg/m3/4H; Rat route unknown LD50: 480mg/kg; Mouse oral LD50: 220mg/kg; Mouse inhalation LC50: 690 mg/m3/2H; Mouse peritoneal cavity LD50: 50mg/kg; Rabbit oral LD50: 615mg/kg; 2. Other multiple dose toxicity: Rat inhalation TCLo: 1400 μg/m3/4H/22W-I ;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 35.46
2. Molar volume (cm3/mol): 125.2
3. Isotonic specific volume (90.2K ): 290.3
4. Surface tension (dyne/cm): 28.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 14.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 26
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 132
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxidizing agents. Toxic if inhaled or in contact with skin, harmful if taken orally, and has cumulative hazards.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Reduction of m-nitrotrifluorotoluene with iron powder: Add hydrochloric acid and iron powder to water in sequence, heat to 80°C, slowly add m-nitrotrifluorotoluene dropwise, and control the addition at 80-85°C. Raise the temperature to reflux within 1.5 hours, keep it warm for 3 hours, cool to 50°C, add sodium carbonate solution (10%) 1.7 times, distill, remove the water layer from the distillate, dry the oil with anhydrous sodium sulfate and filter, to obtain Aminobenzotrifluoride. The yield is 85.7%.
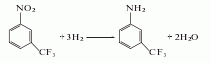
2. The preparation method is: Add m-trifluoromethylnitrobenzene, solvent ethanol and catalyst to the autoclave in turn, replace the air in the autoclave with nitrogen, start stirring, and add hydrogen to keep the pressure in the autoclave at about 0.1 MPa and control the temperature at (100± 5)℃, react until no hydrogen is absorbed, the reaction time is about 1 hour, cool it with cooling water, so that the temperature in the kettle drops below 40℃, use the residual pressure in the kettle to discharge the material, filter the reaction liquid, remove the catalyst, and evaporate under normal pressure Remove the solvent and water, and distill under reduced pressure to obtain the finished product.
Purpose
1. Used in organic synthesis and dye intermediates.
2. It is used to prepare the antipsychotic drugs fluphenazine, trifluperazine, trifluoperazine, etc.; it is also used to synthesize the herbicides fluridone, fluroturone, etc. To control broadleaf weeds and perennial weeds in wheat and cotton fields.



 微信扫一扫打赏
微信扫一扫打赏
