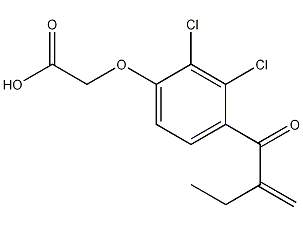
Structural formula
| Business number | 019H |
|---|---|
| Molecular formula | C13H12Cl2O4 |
| Molecular weight | 303.14 |
| label |
2,3-Dichloro-4-(2-methylenebutyryl)phenoxyacetic acid, (2,3-Dichloro-4-[2-methylenebutyryl]phenoxy)acetic acid |
Numbering system
CAS number:58-54-8
MDL number:MFCD00056693
EINECS number:200-384-1
RTECS number:AG6600000
BRN number:None
PubChem number:24894577
Physical property data
1. Appearance: White crystalline powder
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL , air=1): Undetermined
4. Melting point (ºC): 121~125
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Very soluble in ethanol, soluble in ether, chloroform or glacial acetic acid, insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 72.41
2. Molar volume (cm3/mol): 224.4
3. Isotonic specific volume (90.2K ): 586.9
4. Surface tension (dyne/cm): 46.8
5. Polarizability (10-24cm3): 28.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.8
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 63.6
7. Number of heavy atoms: 19
8. Surface charge: 0
9. Complexity: 370
10. Number of isotope atoms: 0
11. Determined number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13.�The number of stereocenters of definite chemical bonds: 0
14. The number of stereocenters of uncertain chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
None
Storage method
Keep in a sealed container away from light.
Synthesis method
2,3-Dichloroanisole is condensed with butyryl chloride and hydrolyzed into 2,3-dichloro-4-butyrylphenol, which is then condensed with ethyl bromoacetate, and then further condensed with formaldehyde and hydrolyzed into ethainide acid. 1.2. Preparation of 3-dichloro-4-butyrylphenol. Add butyryl chloride, dichlorobenzomethyl ether and aluminum trichloride to carbon disulfide in sequence, react at room temperature for 1 hour, and then recycle carbon disulfide after flowing for another 1 hour. After recovery is completed, add n-heptane, then add aluminum trichloride, and continue refluxing for 3 hours. Cool to 50°C, separate the n-heptane, cool, add dilute hydrochloric acid, raise the temperature to 86°C, immediately cool to 40°C, filter and wash to obtain crude 2,3-dichloro-4-butyrophenol. The crude product is refined by acid-base method and decolorized with activated carbon to obtain a fine product with a melting point of 108-111°C. The yield is 85%. 2. Preparation of ethacrynic acid: Heat 2,3-dichloro-4-butyrophenol, absolute ethanol and sodium acetate to reflux, add ethyl bromoacetate dropwise, and recover the ethanol after 3 hours of reaction. Cool, filter, wash, and recrystallize with ethanol to obtain ethyl 2,3-dichloro-4-butyrylphenoxyacetate. Then put it into a mixture of formaldehyde and ethanol, add a mixture of potassium carbonate, water and ethanol dropwise under reflux, and continue refluxing for 1.5 hours. Cool, add dilute hydrochloric acid to precipitate, filter, wash, dry, and finally undergo recrystallization and activated carbon decolorization to obtain ethacrynic acid. The yield is about 40%.
Purpose
This product is a strong diuretic. Acts quickly but short-lived. It has the effect of inhibiting the reabsorption of sodium and chloride ions by the renal tubules. It is a necessary drug for severe edema and is used for many types of edema. However, it is highly toxic and should be used sparingly in patients with chronic renal insufficiency.



 微信扫一扫打赏
微信扫一扫打赏
