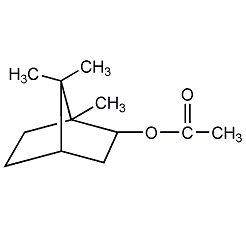
Structural formula
| Business number | 01L3 |
|---|---|
| Molecular formula | C12H20O2 |
| Molecular weight | 196.29 |
| label |
Endo-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol acetate, 1-Bornyl acetate, Bornyl acetate, endo-1,7,7-Trimethylbicyclo[2.2.1]hept-2-yl acetate, 2-Camphanol acetate, endo-2-Camphanyl ethanoate, Bornyl acetic ether, Borneol acetate |
Numbering system
CAS number:76-49-3
MDL number:MFCD00135944
EINECS number:200-964-4
RTECS number:None
BRN number:None
PubChem number:24900675
Physical property data
1. Physical property data
1. Characteristics: colorless liquid. It has a strong pine-like cool aroma and spicy taste.
2. SecretDegree (g/mL,25/4℃):0.991g/cm3
3. Relative vapor density (g/mL,AIR=1): Unsure
4. Melting point (ºC): 27.7 ℃
5. Boiling point (ºC,Normal pressure):225-226℃
6. Boiling point (ºC,5.2kPa): Unsure
7. Refractive index:1.4639
8. Flash Point (ºC): Unsure
9. Specific optical rotation (º): right-handed[α]D+44.38°, optical rotation[α]D+41.2°;Levorotaryα]D-44.45°, optical rotation[α]D-42.0°
10. Autoignition point or ignition temperature (ºC 1. Molar refractive index: 55.34
2. Molar volume (m3/mol):194.7
3. isotonic specific volume (90.2K):465.6
4. Surface Tension (dyne/cm):32.6
5. Polarizability(10-24cm3): 21.94
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 270
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 3
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
Mainly uses esterification method. It is obtained by using tetracene and acetic acid as raw materials, carrying out esterification reaction in the presence of sulfuric acid, activated clay or ion exchange resin catalyst, and then neutralizing, washing, drying and distilling. It can also be extracted from natural raw materials such as Canadian hemlock, white fir, and red pine.
Purpose
Ester synthetic fragrances. It is mainly used as a blending spice for green-flavored and fougere-flavored flavors, and is used to prepare room spray flavors, bath flavors, etc.
Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed and stored in a cool, dry place away from light.
Synthesis method
Mainly uses esterification method. It is obtained by using tetracene and acetic acid as raw materials, carrying out esterification reaction in the presence of sulfuric acid, activated clay or ion exchange resin catalyst, and then neutralizing, washing, drying and distilling. It can also be extracted from natural raw materials such as Canadian hemlock, white fir, and red pine.
Purpose
Ester synthetic fragrances. It is mainly used as a blending spice for green-flavored and fougere-flavored flavors, and is used to prepare room spray flavors, bath flavors, etc.



 微信扫一扫打赏
微信扫一扫打赏
