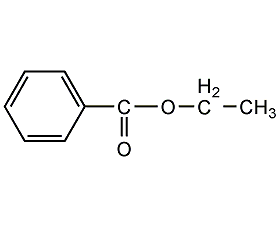
Structural formula
| Business number | 026V |
|---|---|
| Molecular formula | C9H10O2 |
| Molecular weight | 150.17 |
| label |
Ethyl benzoate, Ethylbenzoic acid, Benxoic acid ethyl ester, spices, aromatic compounds |
Numbering system
CAS number:93-89-0
MDL number:MFCD00009109
EINECS number:202-284-3
RTECS number:DH0200000
BRN number:1908172
PubChem number:24894400
Physical property data
1. Properties: Colorless and transparent liquid, with floral and fruity aroma like ylang ylang, milder than methyl benzoate.
2. Boiling point (ºC): 211~214, 87ºC (1.3kpa)
3. Melting point (ºC): -34
4. Relative density (d204) : 1.05 (20℃)
5. Relative density: 1.0458 (25℃)
6 . Refractive index (20℃): 1.5006
7. Refractive index (15℃): 1.5205
8. Flash point (ºC, closed): 95.5
9. Flash point (ºC, open): 84
10. Fire point (ºC): 644
11. Heat of vaporization (KJ/mol): 40.49
12. Heat of combustion (KJ/mol): 4602.6
13. Specific heat capacity (KJ/(kg·K), 15~30ºC, constant pressure): 1.61
14. Electrical conductivity (S/m, 19ºC): <2×10-10
15. Thermal conductivity (W/(m·K), 32ºC): 0.16
16. Lower explosion limit (%, V/V): 1
17. Volume expansion coefficient (K-1): 0.00089
18. Solubility: Slightly soluble in water, soluble in ethanol and ether.
19. Liquid phase standard hot melt (J·mol-1·K-1): 245.0
Toxicological data
It is slightly toxic. It can cause coughing, and the oral LD50 in rats is 6.5g/kg. Rats inhaled saturated vapor for 8 hours without death.
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 42.65
2. Molar volume (cm3/mol): 143.8
3. Isotonic specific volume (90.2K ): 351.9
4. Surface tension (dyne/cm): 35.8
5. Polarizability (10-24cm3): 16.91
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:��
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 126
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenter Number of stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Total Number of price key units: 1
Properties and stability
1. Chemical properties: The chemical properties of ethyl benzoate are relatively stable and hydrolyze in the presence of caustic alkali to produce benzoic acid and ethanol. It is partially decomposed when heated to 305°C in a sealed tube. It decomposes under electric spark to produce acetylene, hydrogen, carbon monoxide, carbon dioxide and a small amount of methane. When heated to 400°C in the presence of thorium oxide, benzoic acid and ethylene are produced. Ethyl benzoate and sodium ethoxide are relatively stable when heated to 120°C. But it decomposes into sodium benzoate and ether at 160℃. Ethyl benzoate reacts with chlorine at 200°C to obtain benzoyl chloride and a small amount of acetyl chloride. Heated with bromine to 170~270℃ to generate benzoic acid and vinyl bromide. Reacts with phosphorus pentachloride at 140°C to produce ethyl chloride and benzoyl chloride.
2. When ethyl benzoate and nitric acid undergo nitration reaction at room temperature, 3-nitrobenzoate ethyl ester and 2- or 4-nitrobenzoate ethyl ester are produced in a ratio of 2:1. . Ethyl benzoate reacts with lithium aluminum hydride in an ether solution to form benzyl alcohol. When heated to about 500°C with ammonia in the presence of alumina or thorium oxide, benzonitrile and ethylene are generated. Reacts with ammonia at 200°C to form benzamide. Ethyl benzoate can form crystalline complexes with various metal salts and tin chloride, aluminum trichloride, titanium chloride, magnesium iodide, antimony pentachloride, etc. Most of these crystalline complexes are unstable. , easily decomposes in the air.
3. Exist in flue-cured tobacco leaves.
4. Naturally found in peaches, pineapples, and black tea.
Storage method
Pay attention to fire sources and store in a cool and ventilated place. Iron, mild steel or aluminum containers can be used for storage, but copper containers are not suitable.
Synthesis method
1. Mainly adopt esterification method. It is obtained by using benzoic acid and ethanol as raw materials, carrying out esterification reaction in the presence of concentrated sulfuric acid catalyst, and then neutralizing, washing and distilling. It can also be obtained by using methyl benzoate and ethanol as raw materials and carrying out transesterification reaction in the presence of potassium ethoxide.
2.It is obtained by direct esterification of benzoic acid and ethanol and distillation.
Refining method: often contains impurities such as free acid and alcohol. During refining, wash with potassium bicarbonate or sodium carbonate solution, dry with anhydrous potassium carbonate or sodium sulfate and then rectify.
3. Tobacco: FC, 18.
Purpose
1. Used for preparing ylang-ylang flavor for soap; also used as solvent for cellulose ester, cellulose ether, resin, etc.
2.Can be used as food flavor to prepare bananas, strawberries, peaches, cherries, grapes, vanilla, walnuts, black currants, etc. The dosage used in gum is 59mg/kg; in baked goods it is 10mg/ kg; 9.0mg/kg in candies; 2.8mg/kg; 2.8mg/kg in cold drinks. Also used in wine flavors and tobacco flavors.



 微信扫一扫打赏
微信扫一扫打赏
