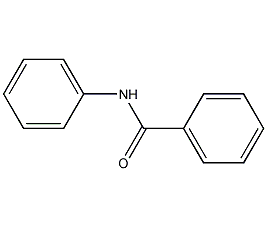
Structural formula
| Business number | 026Z |
|---|---|
| Molecular formula | C13H11NO |
| Molecular weight | 197.23 |
| label |
N-phenylbenzamide, benzoanilide, N-benzoylanilide, N-phenylinide, Benzoic acid anilide, N-Phenylbenzamide, C6H5CONHC6H5 |
Numbering system
CAS number:93-98-1
MDL number:MFCD00003069
EINECS number:202-292-7
RTECS number:None
BRN number:1102980
PubChem number:24846656
Physical property data
1. Character:Colorless or white flaky crystals or powder. Can be sublimated. Does not decompose during distillation.
2. Density (g/mL, 25/4℃):1.315
3. Relative vapor density (g/ mL,air=1 ): Undetermined
4. Melting point (ºC):163
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,1.33kPa ):117-119
7. Refractive Index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
Product soluble in7mlBoiling ethanol,60ml Cold ethanol. Slightly soluble in ether, insoluble in water.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index:60.82
2. Molar volume (m3/ mol):168.3
3. Isotonic specific volume (90.2K): 446.8
4. Surface tension (dyne/cm): 49.7
5. Polarizability(10-24cm3):24.11
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 203
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
Derived from the reaction of aniline and benzoic acid. React dry benzoic acid and freshly steamed aniline at 180-190°C for 2 hours, then increase the temperature to 225°C. At the beginning, water is distilled off during the reaction, then aniline is distilled off, and finally all the fractions are distilled off. The fractions evaporated after 255°C are cooled, solidified, and ground to obtain a purple-grey crude product. The crude product is washed with dilute hydrochloric acid to remove remaining aniline, washed with water and then added with sodium hydroxide solution to remove remaining benzoic acid. Finally, wash with water, filter, dry, and recrystallize with ethanol to obtain a refined finished product.
Purpose
Dye, fragrance and pharmaceutical intermediates.
>Polarizability(10-24cm3):24.11
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 29.1
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 203
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be kept sealed.
Synthesis method
Derived from the reaction of aniline and benzoic acid. React dry benzoic acid and freshly steamed aniline at 180-190°C for 2 hours, then increase the temperature to 225°C. At the beginning, water is distilled off during the reaction, then aniline is distilled off, and finally all the fractions are distilled off. The fractions evaporated after 255°C are cooled, solidified, and ground to obtain a purple-grey crude product. The crude product is washed with dilute hydrochloric acid to remove remaining aniline, washed with water and then added with sodium hydroxide solution to remove remaining benzoic acid. Finally, wash with water, filter, dry, and recrystallize with ethanol to obtain a refined finished product.
Purpose
Dye, fragrance and pharmaceutical intermediates.



 微信扫一扫打赏
微信扫一扫打赏
