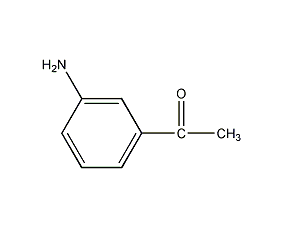
Structural formula
| Business number | 02F1 |
|---|---|
| Molecular formula | C8H9NO |
| Molecular weight | 135.16 |
| label |
m-acetanilide, m-aminoacetophenone, 3-Acetylaniline, 3-Aminoacetophenone, 1-(3-Aminophenyl)-ethanone, M-acetylaniline, m-Aminophenylmethyl ketone |
Numbering system
CAS number:99-03-6
MDL number:MFCD00007796
EINECS number:202-722-3
RTECS number:AM5800000
BRN number:386009
PubChem number:24845944
Physical property data
1. Properties: yellow leaf-like crystals.
2. Density (g/mL, 25℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 98-99
5. Boiling point (ºC, normal pressure): 289-290
6. Boiling point (ºC, 30mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20ºC): Not determined
12. Saturated vapor Pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in alcohol, insoluble in water.
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit skin contact, 500mg/24HREACTION SEVERITY, slight reaction; 2. Acute toxicity: rat oral LD50: 1870mg/kg; mouse peritoneal cavity LD50: 2048mg/kg ; Rabbit skin contact LD50: 4340μL/kg; 3. Mutagenicity: Mutant microbial test: bacteria-Salmonella typhimurium, 10mg/L; Cell generation analysis test: mouse peritoneal cavity, 400mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Moore’s foldEmissivity: 40.51
2. Molar volume (cm3/mol): 123.2
3. Isotonic specific volume (90.2K): 318.2
4. Surface tension (dyne/cm): 44.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.06
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.2
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecular polar surface area (TPSA): 43.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms : 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides and acids.
2. It is irritating and harmful if taken orally. Avoid inhaling the dust of this product and avoid contact with eyes and skin.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants and acidic substances, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
It is obtained by reducing m-nitroacetophenone with iron powder. Add water to the reaction tank, add iron powder under stirring, and slowly add industrial hydrochloric acid. Then add m-nitroacetophenone in batches. Heating and refluxing for 6h. Cool to 30°C and add hydrochloric acid to make the reaction solution strongly acidic. Filter, add ammonia water to the filtrate until it becomes weakly alkaline, and solid matter will precipitate. After filtering, the filter cake is m-aminoacetophenone. The yield is 85%.
Purpose
1. Pharmaceutical intermediates. It can also be used as raw material for organic synthesis.
2. Synthesis of spices and reagents for detecting acetoacetate.



 微信扫一扫打赏
微信扫一扫打赏
