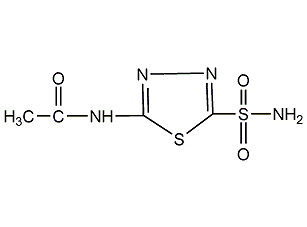
Structural formula
| Business number | 01AL |
|---|---|
| Molecular formula | C4H6N4O3S2 |
| Molecular weight | 222.25 |
| label |
5-Acetamido-1,3,4-thiadiazole-2-sulfonamide, N-(5-[Aminosulfonyl]-1,3,4-thiadiazol-2-yl)acetamide, N-(5-Sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide |
Numbering system
CAS number:59-66-5
MDL number:MFCD00003105
EINECS number:200-440-5
RTECS number:AC8225000
BRN number:212994
PubChem number:24278144
Physical property data
1. Appearance: White needle-like crystal or crystalline powder
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 256-261℃ (decomposition)
5. Boiling point (ºC, normal pressure): 304-305
6. Boiling point (ºC, 5.2kPa): Not determined
7. Refractive index: Undetermined
8. Flash point (ºC) : 173
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water (octanol/water) distribution Log value of coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined 19. Solubility : Slightly soluble in boiling water, slightly soluble in water and ethanol, almost insoluble in chloroform or ether, easily soluble in ammonia solution.
Toxicological data
Acute toxicity
Main irritant effects:
On skin: May cause inflammation
On eyes: May cause inflammation
Sensitization: No known sensitizing effects.
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is extremely hazardous to water.
Do not allow undiluted or large amounts of product to come into contact with groundwater, waterways or sewage systems.
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 45.95
2. Molar volume (cm3/mol): 127.3
3. Isotonic specific volume (90.2K): 400.7
4. Table�� Tension (dyne/cm): 97.9
5. Polarizability (10-24cm3): 18.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 152
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 297
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
Seal and save.
Synthesis method
1. Chlorination and oxidation of 2-acetylamino-5-mercapto-1,3,4-thiadiazole is added to the mixture of glacial acetic acid and water, cooled to -2°C and passed through chlorine, and the final temperature does not exceed 5°C . When the material temperature drops significantly and a large amount of foam appears on the liquid surface, stop passing chlorine when the liquid chlorine is consumed to the specified amount, filter, and wash the filter cake with ice water until the pH is 4 to obtain oxychloride (2-acetyl chloride-5 -Sulfonyl chloride-1,3,4-thiadiazole). 2. Amination Mix ammonia water with crushed ice and cool down to below 5℃. Add oxychloride, complete the addition, and keep warm for half an hour. Adjust the pH to 5-6 with hydrochloric acid, filter, wash the filter cake until the pH is 7, and dry to obtain crude acetazolamide. Heat the crude product, water, activated carbon and sodium metabisulfite to 95°C, decolorize for 1-1.5 hours, filter while hot, cool the filtrate to crystallize, filter, wash and dry to obtain acetazolamide.
Purpose
Carbonic anhydrase inhibitors.



 微信扫一扫打赏
微信扫一扫打赏
