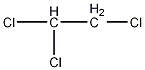
Structural formula
| Business number | 01P2 |
|---|---|
| Molecular formula | C2H3Cl3 |
| Molecular weight | 133.40 |
| label |
β-Trichloroethane, Ethane trichloride, Ethylene trichloride, Vinyl trichloride, β-Trichloroethane, Aliphatic halogenated derivatives |
Numbering system
CAS number:79-00-5
MDL number:MFCD00000852
EINECS number:201-166-9
RTECS number:KJ3150000
BRN number:1731726
PubChem number:24845354
Physical property data
1. Properties: colorless liquid with aromatic odor. [1]
2. Melting point (℃): -36.5[2]
3. Boiling point (℃): 114[3]
4. Relative density (water = 1): 1.44[4]
5. Relative vapor Density (air=1): 4.55[5]
6. Saturated vapor pressure (kPa): 2.5 (20℃)[6]
7. Heat of combustion (kJ/mol): -1097.2[7]
8. Critical pressure (MPa): 4.83[8]
9. Octanol/water partition coefficient: 2.17[9]
10. Ignition temperature (℃): 460[10]
11. Explosion upper limit (%): 13.3[11]
12. Explosion lower limit (%): 8.4 [12]
13. Solubility: Insoluble in water, miscible in alcohols, ethers, esters, ketones, etc. [13]
14. Viscosity (mPa·s, 15ºC): 1.20
15. Heat of evaporation (J/g, b.p.): 287.6
16. Heat of combustion (KJ/mol, gas): 1099.0
17. Specific heat capacity (KJ/(kg·K), 20ºC): 1.13
18 .Thermal conductivity (W/(m·K), 20ºC, liquid): 0.135
19. Volume expansion coefficient (K-1, 0~25ºC, average): 0.0010
20. Relative density (25℃, 4℃): 1.4321
21. Refractive index at room temperature (n25): 1.4689
22.Solubility parameter (J·cm-3)0.5:19.819
23.van der Waals area (cm 2·mol-1): 7.360×109
24. van der Waals volume (cm3 sup>·mol-1): 53.110
25. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -191.5
26. Liquid phase standard hot melt (J·mol-1·K-1): 151.5
27. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -145.6
28. Gas phase standard entropy (J·mol-1· K-1): 333.53
29. Gas phase standard free energy of formation (kJ·mol-1): -83.2
30. Vapor phase standard hot melt (J·mol-1·K-1): 84.89
Toxicological data
1. Acute toxicity[14]
LD50: 836mg/kg (rat oral); 3730mg/kg (rabbit dermal )
2. Irritation [15]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 162mg, mild irritation.
3. Subacute and chronic toxicity[16] Rats, guinea pigs and rabbits inhaled 0.82g/m3, 7 hours a day, 5 days a week, for 6 months, no abnormalities were found; inhaled 1.6g /m3, female rats had mild hepatic steatosis and cellular turbidity.
4. Mutagenicity [17] Micronucleus test: human lymphocytes 100 μmol/L. DNA damage: human lymphocytes 2500 μmol/L. Cytogenetic analysis: Guinea pig skin exposure 2880μg/kg
5. Carcinogenicity [18] IARC carcinogenicity review: G3, for humans and There is insufficient evidence of carcinogenicity in animals.
Ecological data
1. Ecotoxicity[19]
LC50: 81.6mg/L (96h) (fathead minnow, dynamic); 133mg/ L (48h) (medaka)
IC50: 93~430mg/L (72h) (algae)
2. Biodegradability[20]
Aerobic biodegradation (h): 4320~8760
Anaerobic biodegradation (h): 17280~35040
3. Non-biodegradability[21]
Photooxidation half-life in air (h): 196~1956
一 Grade hydrolysis half-life (h): 3.26×105
4. Other harmful effects[22] This substance has May be hazardous to the environment and accumulate in groundwater.
Molecular structure data
1. Molar refractive index: 25.82
2. Molar volume (cm3/mol): 96.0
3. Isotonic specific volume (90.2K ): 224.4
4. Surface tension (dyne/cm): 29.7
5. Polarizability (10-24cm3): 10.23
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 18.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Miscible with general organic solvents such as ethanol, ether, and organic chlorides. Can dissolve grease, wax, natural resin, rubber, ethyl cellulose and vinyl resin, etc. The solubility in water at 20°C is 0.436%; the solubility of water in 1,1,2-trichloroethane is 0.05%. Dry 1,1,2-trichloroethane is not very corrosive to metals. Contact with moist air, especially under light, releases highly corrosive hydrogen chloride. Liquid 1,1,2-trichloroethane has a strong degreasing effect and should be avoided in contact with skin.
2. Stable properties under normal conditions. In the absence of air and water, no obvious decomposition will occur when heated to 110°C, but hydrolysis will occur when it comes into contact with water at its boiling point. Heated with sodium hydroxide solution or calcium hydroxide suspension, 1,1-dichloroethylene is generated, and dehydrochlorination reaction also occurs in high-temperature gas phase thermal cracking. In the presence of aluminum trichloride, it reacts with chlorine at 70~80°C to form 1,1,2,2-tetrachloroethane.
3. Stability[23] Stable
4. Incompatible substances[24] Strong alkali, strong oxidant, aluminum, magnesium
5. Conditions to avoid contact [25] Humid air, Light
6. Polymerization hazard[26] No polymerization
7. Decomposition products[27 ] Hydrogen chloride
Storage method
Storage Precautions[28] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Vinyl chloride chlorination method: Put trichloroethane into the reaction kettle in advance, and then introduce vinyl chloride and chlorine gas (molar ratio 1:1.2) at 20-25°C for chlorination synthesis. The product is obtained by washing with water and separating.
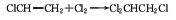
2, 1,2-two Ethyl chloride chlorination method can be obtained by chlorination at 60°C in the presence of aluminum trichloride or other metal chlorides.
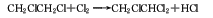
Purpose
1. Used as a solvent for fats, oils, waxes and resins, an extractant for dyes and spices, an intermediate for resins and rubber, agricultural pesticides, fumigants and the synthesis of 1,1-dichloroethylene. of raw materials. Used in the production of vinylidene chloride. Also used as a solvent for cellulose acetate, natural rubber, and chlorinated rubber.
2. Used as solvent for organic synthesis. [29]



 微信扫一扫打赏
微信扫一扫打赏
