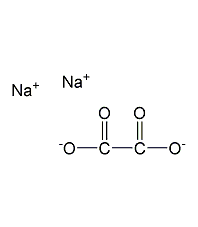
Structural formula
| Business number | 01CN |
|---|---|
| Molecular formula | C2O4Na2 |
| Molecular weight | 134.00 |
| label |
disodium oxalate, sodium oxalate, Ethanedioic acid sodium salt, Oxalic acid disodium salt, Disodium oxalate, finishing agent, luminescent agent, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:62-76-0
MDL number:MFCD00012465
EINECS number:200-550-3
RTECS number:KI1750000
BRN number:3631622
PubChem number:24897926
Physical property data
1. Properties: White crystalline powder. Odorless. Hygroscopic.
2. Density (g/mL, 25/4℃): 2.34
3. Relative vapor density (g/mL, air=1): 3.2
4. Melting point (ºC): 250-257 (decomposition)
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa) : Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12 . Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
p>
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Dissolved in water (37 g/ L at 20 ºC), insoluble in ethanol. Its aqueous solution is nearly neutral.
Toxicological data
Acute toxicity: Human intravenous LDLo: 17 mg/kg; Mouse intraperitoneal LC50: 155 mg/kg; Mouse subcutaneous injection LCLo: 100 mg/kg; Cat subcutaneous injection LDLo: 100 mg/kg Irritant
Ecological data
None yet
Molecular structure data
None yet
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular polarity tableProduct 80.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 60.5
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13 .Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 3
Properties and stability
1. It has strong reducibility. Burning decomposes into sodium carbonate and carbon monoxide. It decomposes into sodium carbonate when heated to above 400°C. Its solubility in 100g of water is 3.7g (20°C) and 6.33g (100°C). It is insoluble in ethanol and ether.
Storage method
1. This product is sealed and stored in a cool, dry place. It can be packed in a plastic bag lined with a lining or a woven bag with an outer cover. Store and transport according to general chemical regulations.
Synthesis method
1. Carbon monoxide and sodium hydroxide react at 160°C and 2MPa to generate sodium formate. Then, sodium formate is dehydrogenated at 400°C to obtain sodium oxalate.
![]()
2. Take 212g of sodium carbonate and heat it Dissolve in 1L of water, and add a small amount of oxalic acid aqueous solution to the aqueous solution. At this time, while sodium oxalate precipitates, the impurities in it can be co-precipitated with sodium oxalate and removed. Then take 252g of refined oxalic acid and dissolve it in 1L of hot water (90°C). In a beaker, heat 500mL of water to 90°C in advance. While stirring, use a separatory funnel to slowly add equal volumes of oxalic acid and sodium carbonate aqueous solution. Drop it into the beaker. During this process, the pH value of the solution should be maintained at 6 to 7. After the dripping is completed, the solution is slightly heated, and the solution is cooled and the resulting crystals are filtered out and washed with cold water. The crystals are dried at 240 to 250°C and placed in a desiccator with concentrated sulfuric acid.
Refining sodium oxalate. The sodium oxalate prepared according to the above method or commercially available can be purified by recrystallization with water. Dissolve in hot water first, reach saturation at about 90°C, filter while hot, then add 75% (volume fraction) ethanol aqueous solution to the filtrate, let cool, filter out the crystals, and dry at 250°C. Since sodium oxalate aqueous solution can react with glassware, and because the raw materials contain impurities, the product contains silicon dichloride and sodium carbonate. It is recommended that the preparation of sodium oxalate should be done as soon as possible. Refined sodium hydroxide can also be used instead of carbonic acid. sodium. In addition, when preparing sodium oxalate, if the solution is too acidic, a trace amount of sodium hydrogen oxalate will be generated, which will co-precipitate with sodium oxalate. If the product is dried at 250-300°C, sodium hydrogen oxalate will thermally decompose into sodium carbonate.
3.Dissolve oxalic acid dihydrate in water, heat, add sodium hydroxide solution drop by drop to precipitate sodium oxalate crystals, then add water and anhydrous in turn Wash with ethanol and dry to obtain pure sodium oxalate.
4.Slowly add the sodium hydroxide solution to the oxalic acid aqueous solution at 70~80℃ with constant stirring until the phenolphthalein becomes strong. Alkaline reaction, keeping the temperature constant:

After the reaction, quickly filter out the sodium oxalate crystals, wash with water until the pH value is qualified, wash with a small amount of ethanol, and filter with strong suction, and then continue stirring , dry it at 150~200℃.
Purpose
1. Mainly used as an intermediate for the production of oxalic acid, and can also be used in cellulose finishing agents, textiles, leather processing, etc. In analytical chemistry, it is used as a benchmark for calibrating potassium permanganate solution. Firework yellow luminous agent. Standard for calibrating potassium permanganate solution. 2.Benchmark reagent for calibrating potassium permanganate solution. It is also a metal precipitant, reducing agent, complexing agent, masking agent, fabric and tanning finishing agent. 3.Used as a masking agent for leather processing, it can enhance the alkali resistance of the complex. Not easy to settle. 4.Can be used as a reagent and a remover for stains and ink stains.



 微信扫一扫打赏
微信扫一扫打赏
