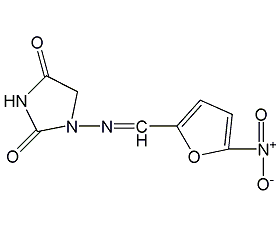
Structural formula
| Business number | 01EH |
|---|---|
| Molecular formula | C8H6N4O5 |
| Molecular weight | 238.16 |
| label |
N-(5-Nitro-2-furfurylidene)-1-aminohydantoin, Furadoxyl |
Numbering system
CAS number:67-20-9
MDL number:MFCD00003224
EINECS number:200-646-5
RTECS number:MU2800000
BRN number:893207
PubChem number:24897842
Physical property data
1. Character:Orange-yellow needle-like crystals, darkening when exposed to light, odorless, bitter taste
2. Density (g/mL,25/4℃): Unsure
3. Relative vapor density (g /mL,Air= 1): Unsure
4. Melting point (ºC):270-272 (decomposed).
5. Boiling point (ºC,Normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Unsure
7. Refractive index:Not sure
8. Flash point (ºC): Unsure
9. Specific optical rotation (º): Unsure
10. Autoignition point or ignition temperature (ºC): Unsure
11. Vapor pressure (kPa,25ºC): Unsure
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index:53.13
2. Molar volume (m3/mol):131.0
3. isotonic specific volume (90.2K):403.2
4. Surface Tension (dyne/cm):89.5
5. Polarizability(10-24cm3):21.06
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 5
6. Topological molecule polar surface area 121
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 390
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be stored in a sealed, cool place away from light.
Synthesis method
Aminohydantoin can be made from hydrazine hydrate and urea, and then condensed with diethyl nitrofuran.
Purpose
Organic synthesis and biochemical research. medicine.
This product is an excellent antibacterial drug, Broad antibacterial spectrum,After oral administration, it is rapidly and completely absorbed and excreted quickly. The amount excreted in urine can reach 40-50 %, commonly used to treat urinary tract infections caused by various sensitive bacteria. It is effective against both Gram-negative and Gram-positive bacteria. It is mainly used for urinary tract infections caused by sensitive bacteria, especially for acute urinary tract infections caused by Escherichia coli and Proteus. Clinically used for urinary system infections caused by sensitive bacteria, such as pyelonephritis, urinary tract infection, cystitis and prostatitis.
��Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 1
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be stored in a sealed, cool place away from light.
Synthesis method
Aminohydantoin can be made from hydrazine hydrate and urea, and then condensed with diethyl nitrofuran.
Purpose
Organic synthesis and biochemical research. medicine.
This product is an excellent antibacterial drug, Broad antibacterial spectrum,After oral administration, it is rapidly and completely absorbed and excreted quickly. The amount excreted in urine can reach 40-50 %, commonly used to treat urinary tract infections caused by various sensitive bacteria. It is effective against both Gram-negative and Gram-positive bacteria. It is mainly used for urinary tract infections caused by sensitive bacteria, especially for acute urinary tract infections caused by Escherichia coli and Proteus. Clinically used for urinary system infections caused by sensitive bacteria, such as pyelonephritis, urinary tract infection, cystitis and prostatitis.



 微信扫一扫打赏
微信扫一扫打赏
