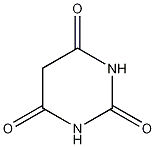
Structural formula
| Business number | 01ES |
|---|---|
| Molecular formula | C4H4N2O3 |
| Molecular weight | 128.09 |
| label |
Heterocyclic compounds |
Numbering system
CAS number:67-52-7
MDL number:MFCD00006666
EINECS number:200-658-0
RTECS number:CP8000000
BRN number:120502
PubChem number:24847314
Physical property data
1. Properties: White crystalline powder. Strongly acidic. Forms salts when meeting metals.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 248 (decomposition)
5. Boiling point (ºC, normal pressure): 260 (decomposition)
6. Boiling point (ºC, 5.2 kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot water And dilute acid, soluble in ether, slightly soluble in cold water.
Toxicological data
Acute toxicity: rat oral LD50: >5 mg/kg; mouse intraperitoneal LD50: 505 mg/kg;
Ecological data
General remarks
Water hazard level 1 (German regulations) (self-assessment via list) This substance is extremely hazardous to water.
Do not allow undiluted or large quantities of product to come into contact with groundwater, waterways or sewage systems
Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 25.53
2. Molar volume (cm3/mol): 88.0
3. Isotonic specific volume (90.2K ): 232.5
4. Surface tension (dyne/cm): 48.7
5. Polarizability (10-24cm3):10.12
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds��:0
5. Number of tautomers: 10
6. Topological molecule polar surface area 75.3
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 168
10. Number of isotope atoms: 0
11. Determine Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
Storage method
This product should be kept sealed. This product is lined with polyethylene film bags and is packaged in iron drums or cardboard drums for export. The net weight of each drum is 25kg.
Synthesis method
Obtained from the reaction of diethyl malonate and urea. First, add urea into a reaction tank containing methanol and heat it to reflux to dissolve, then add dried diethyl malonate and sodium methoxide, and reflux for 4-5 hours at 66-68°C. After distilling and recovering the methanol, wait until it is cooled to 40-50℃, add dilute hydrochloric acid to adjust the pH to 1-2. Let cool to room temperature, throw out the crude product, rinse once with distilled water, spin dry to get the crude product, then refine with water and activated carbon, and spin dry to get the finished product. Industrial product barbituric acid is white or pink crystalline powder, which is highly acidic, with a content of more than 98% and a melting point of ≥245°C. Raw material consumption quota: diethyl malonate 1098kg/t, urea 476kg/t, hydrochloric acid (reagent level III) 681kg/t, sodium methoxide (28%) 369kg/t, methanol 1025kg/t.
![]()
Purpose
Colorimetric determination of furfurals and polypentosides. Organic Synthesis. Used as organic synthesis raw materials, medicines, plastics and dye intermediates. It is an intermediate for barbiturates, vitamin B12 and other drugs. It can also be used as a polymerization catalyst and for the preparation of benzimidazolone organic pigments. It can also be used as a reagent for the colorimetric determination of uronic aldehydes and polypentosides.



 微信扫一扫打赏
微信扫一扫打赏
