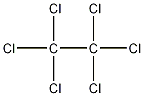
Structural formula
| Business number | 01EZ |
|---|---|
| Molecular formula | C2Cl6 |
| Molecular weight | 236.74 |
| label |
Aliphatic halogenated derivatives |
Numbering system
CAS number:67-72-1
MDL number:MFCD00000799
EINECS number:200-666-4
RTECS number:KI4025000
BRN number:1740341
PubChem number:24869897
Physical property data
1. Characteristics: colorless crystals with a camphor-like odor. [1]
2. Melting point (sublimation, ℃): 186[2]
3. Boiling point (℃) : 186 (103.34kPa, sublimation) [3]
4. Relative density (water = 1): 2.09[4]
5. Relative vapor density (air=1): 8.16[5]
6. Saturated vapor pressure (kPa): 0.13 (32.7℃)[ 6]
7. Critical pressure (MPa): 3.34[7]
8. Octanol/water partition coefficient: 3.82~ 4.19[8]
9. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, benzene, chloroform, oil, etc. [9]
10. The gas phase standard claims heat (enthalpy) (kJ·mol-1): -138.9
11. Gas phase standard entropy (J·mol-1·K-1): 398.85
12. Gas phase standard formation free energy (kJ·mol -1): -54.9
13. Gas phase standard hot melt (J·mol-1·K-1 ): 136.73
14. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -187.6
15. Liquid phase standard entropy (J·mol-1·K-1): 245.6
16. Liquid phase standard formation free energy (kJ·mol– 1): -83.47
17. Liquid phase standard hot melt (J·mol-1·K-1): 196.2
18. Heat of evaporation (KJ/kg, b.p.): 194.2
19. Heat of combustion (KJ/mol, 20ºC, solid): 461
20 . Heat of formation (KJ/mol, 20ºC, crystallization): 226
21. Heat of formation (KJ/mol, 20ºC, gas): 154.9
22. Specific heat capacity (KJ/( kg·K), 25ºC, constant pressure): 0.729
Toxicological data
1. Acute toxicity[10] LD50: 4460mg/kg (rat oral)
2. Irritation No information available
3. Mutagenicity[11] Sister Chromatid exchange: Hamster ovary 330mg/L
4. Carcinogenicity[12] IARC carcinogenicity comments: G2B, suspected human carcinogen.
Ecological data
1. Ecotoxicity[13]
LC50: 0.98mg/L (96h) (rainbow trout , dynamic); 1.53mg/L (96h) (fathead minnow, dynamic); 2.9mg/L (48h) (water flea)
2. Biodegradability[14]
Aerobic biodegradation (h): 672~4320
Anaerobic biodegradation (h): 2688 ~17280
3. Non-biodegradability No data yet
4. Other harmful effects[15] This substance may be harmful to the environment and may accumulate in groundwater. In the oppositeBioaccumulation occurs in important food chains, especially in aquatic organisms.
Molecular structure data
1. Molar refractive index: 40.34
2. Molar volume (cm3/mol): 129.9
3. Isotonic specific volume (90.2K ): 332.5
4. Surface tension (dyne/cm): 42.8
5. Polarizability (10-24cm3): 15.99
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 61.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Hexachloroethane can sublimate and gradually volatilize in the air. Chemically stable, it will not decompose when in contact with water at room temperature. It also does not react in acid or alkali aqueous solutions. Reacts with zinc chloride to form carbon tetrachloride. Reduction with zinc and sulfuric acid produces tetrachlorethylene. High temperature pyrolysis generates carbon tetrachloride, tetrachlorethylene and chlorine.
2. Stability[16] Stable
3. Prohibited use Objects[17] Strong oxidants, strong alkali, metals, etc.
4. Conditions to avoid contact[18] Light and heat
5. Polymerization hazard[19] No polymerization
6. Decomposition products[20] Hydrogen chloride, phosgene
Storage method
Storage Precautions[21] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, alkalis, and food chemicals, and avoid mixed storage. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Obtained from chlorination of tetrachloroethane. Put the dehydrated tetrachloroethane into a glass-lined reaction pot, pass in chlorine gas under strong light, and the reaction temperature gradually rises, and is finally maintained at 90-100°C. When the reaction liquid turns from turbid to clear and crystallization appears in the chlorine pipe, chlorination has reached the end point. Stop passing chlorine, increase the temperature to 120°C, and discharge the remaining chlorine in the pot to the end. Stop passing chlorine, raise the temperature to 120°C, and discharge the remaining chlorine in the pot. Discharge the material into a crystallization pot filled with boiling water, wash with hot water, add 1% sodium carbonate and 5% urea solution and stir to remove chlorine. After being fully washed with water, the crystals are cooled and filtered by centrifugation. Finally, the crystals are soaked in distilled water and then dried, dried at 40°C, and crushed to obtain hexachloroethane. Trichlorethylene and chlorine are also used in industry for photochlorination. Trichlorethylene reacts with chlorine to form pentachloroethane, which reacts with liquid alkali to form tetrachlorethylene. The crude product is obtained by photochlorination reaction between tetrachlorethylene and liquid chlorine (1:0.213), which is then neutralized with soda ash, washed with water, cooled and crystallized, centrifugally filtered, dried and crushed to obtain the finished product. Using this method to produce hexachloroethane consumes 680kg of trichlorethylene and 700kg of liquid chlorine per ton of product. In addition, this product can be produced by reacting carbon tetrachloride with chlorine in the presence of aluminum chloride, or reacting ethylene with chlorine at 300-350°C.
Purpose
1. This product is used as a solvent and in the production of Freon-113, pesticides, medicines, veterinary drugs, smoking agents, defoaming agents, degassing agents and deoxidants for aluminum products (aluminum, magnesium, copper and their Alloys), cutting oil additives, polyvinyl chloride co-plasticizers, and organic synthesis intermediates. Hexachloroethane can be used as a smoke generating agent when mixed with zinc powder, ammonium chloride, ammonium perchlorate, etc. in proportion. Hexachloroethane is also used as a camphor substitute and rubber vulcanization accelerator. Hexachloroethane itself is also a veterinary anthelmintic. Mainly used for ruminants liver leech disease and gastric leech disease, other orchiasis and nematode diseases, etc.
2. Used in organic synthesis, pharmaceutical industry, etc. Also used as solvent. [22]



 微信扫一扫打赏
微信扫一扫打赏
