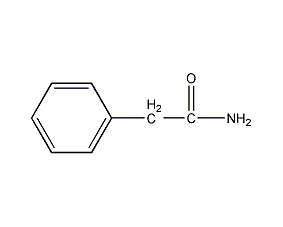
Structural formula
| Business number | 02P9 |
|---|---|
| Molecular formula | C8H9NO |
| Molecular weight | 135 |
| label |
2-phenylacetamide, 2-Phenylacetamide, a-Toluimidic acid, Phenylacetic acid amide |
Numbering system
CAS number:103-81-1
MDL number:MFCD00059193
EINECS number:203-147-0
RTECS number:AC7705000
BRN number:507886
PubChem ID:None
Physical property data
1. Character: white flake or leaf-like crystal
2. Density (g/mL, 20℃): 1.0179
3. Relative vapor density (g/mL , air=1): Undetermined
4. Melting point (ºC): 157-158
5. Boiling point (ºC, normal pressure): 280-290 (decomposition)
p>
6. Boiling point (ºC, mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in hot water and ethanol, slightly soluble in cold water, ether and benzene.
Toxicological data
Acute toxicity: mouse peritoneal cavity LD50: 430mg/kg;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 39.36
2. Molar volume (cm3/mol): 123.0
3. Isotonic specific volume (90.2K ): 317.9
4. Surface tension (dyne/cm): 44.5
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.60
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 43.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 116
10. Number of isotope atoms: 0
11.��Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, and strong alkali.
2. Exist in mainstream smoke.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
Put styrene, sulfur, liquid ammonia and water into the autoclave, react at 165℃ and about 6.5MPa pressure, then heat and evaporate to drive away the hydrogen sulfide gas, add activated carbon to decolorize, cool, crystallize, filter and dry. Obtain phenylacetamide. This method can be improved to pipeline continuous production. Mix styrene and ammonium polysulfide liquid in a volume ratio of 1:2, and pass them through a high-pressure pipeline. The reaction temperature is 200°C, the reaction pressure is 6-7.8MPa, and the reaction time is 1.5h. The post-treatment process of the reaction product is similar to the autoclave batch method. Raw material consumption quota: styrene (99%) 1117kg/t, sulfur (98.5%) 716kg/t, liquid ammonia (99%) 539kg/t, activated carbon 47kg/t.
Another production method is to hydrolyze phenylacetonitrile (obtained from the reaction of benzyl chloride and sodium cyanide in dimethylamine aqueous solution) by heating in sulfuric acid or hydrochloric acid. Add phenylacetonitrile to concentrated hydrochloric acid, stir to dissolve, and react at 50°C for half an hour. Then slowly add water to precipitate crystals under cooling, filter after cooling, and wash with ice water to obtain the crude product. The crude product is washed with sodium carbonate solution, then washed with ice water, and after drying, relatively pure phenylacetamide can be obtained. Raw material consumption quota: benzyl chloride (95%) 1190kg/t, sodium cyanide (95%) 527kg/t, dimethylamine (industrial product) 20kg/t, sodium carbonate (industrial product) 185kg/t, sulfuric acid (92.5 %)1760kg/t.
Purpose
Used as an intermediate for drugs such as penicillin and phenobarbital. It is also used in the preparation of phenylacetic acid, spices and pesticide rodenticide raw materials.



 微信扫一扫打赏
微信扫一扫打赏
