
Structural formula
| Business number | 031L |
|---|---|
| Molecular formula | C4H4N2 |
| Molecular weight | 80.09 |
| label |
Succinonitrile, 1,2-dicyanoethane, 1,2-Dicyanoethane, sym-Dicyanoethane, Deprelin, Colorant, Extracting agent, polish, synthetic raw materials, Intermediates |
Numbering system
CAS number:110-61-2
MDL number:MFCD00001949
EINECS number:203-783-9
RTECS number:WN3850000
BRN number:1098380
PubChem ID:None
Physical property data
1. Properties: colorless and odorless waxy solid. [1]
2. Melting point (℃): 54~56[2]
3. Boiling point (℃) :265~267[3]
4. Relative density (water=1): 0.985[4]
5 .Relative vapor density (air=1): 2.8[5]
6. Saturated vapor pressure (kPa): 0.27 (100℃)[6]
7. Heat of combustion (kJ/mol): -2281.0[7]
8. Critical pressure (MPa): 3.54 [8]
9. Octanol/water partition coefficient: -0.99[9]
10. Flash point (℃): 132[10]
11. Ignition temperature (℃): 511[11]
12. Explosion upper limit ( %): 14.4[12]
13. Lower explosion limit (%): 2.05[13]
14. Dissolution Properties: Slightly soluble in water, ethanol, benzene, ether and carbon disulfide, soluble in acetone, chloroform and dioxane. [14]
15. Refractive index (60ºC): 1.41734
16. Viscosity (mPa·s, 60ºC): 2.591
17. Heat of evaporation (KJ/mol, 25ºC): 64.02
18. Heat of fusion (KJ/mol): 3.71
19. Heat of formation (KJ/mol, 25ºC) ): 227.85
20. Specific heat capacity (KJ/(kg·K), 61.85ºC, constant pressure): 2.00
21. Conductivity (S/m): 5.64×10 -4
22. Solubility (%, 25ºC, water): 11.5
Toxicological data
1. Acute toxicity[15]
LD50: 450mg/kg (rat oral); 129mg/kg (mouse Oral)
2. Irritation No information available
3. Others[16] The lowest toxic dose in the abdominal cavity of hamsters (TDLo): 365mg/kg (8 days of pregnancy), causing embryotoxicity and abnormal development of the central nervous system.
Ecological data
1. Ecotoxicity No data yet
2. Biodegradability[17]
Activated sludge method (sludge concentration 2500mg/L), initial concentration 500mg/L, removal rates of 6h, 12h and 24h are 1.5%, 2.4% and 3.8% respectively.
3. Non-biodegradability [18] The half-life of photodegradation in air is 380 days(Theory)
4. Other harmful effects [19] This substance is harmful to the environment, so special attention should be paid to Pollution of water bodies.
Molecular structure data
1. Molar refractive index: 20.40
2. Molar volume (cm3/mol): 81.3
3. Isotonic specific volume (90.2K ): 207.8
4. Surface tension (dyne/cm): 42.6
5. Polarizability (10-24cm3): 8.09
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 47.6
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 92
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[20] Stable
2. Incompatible substances[21] Strong oxidizing agent, strong reducing agent, strong acid, strong alkali
3. Polymerization hazard[22] No polymerization
4. Decomposition products[23] Cyanide
Storage method
1. This product is generally packed in barrels, 180kg per barrel. It should be placed in a cool and ventilated place and stored and transported according to the regulations on dangerous goods.
2. Storage precautions [24] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, reducing agents, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
It is produced by reacting acrylonitrile with hydrocyanic acid under alkaline conditions. The yield is over 95%. The sodium cyanide aqueous solution can also be heated, the ethanol solution of 1,2-dibromoethane can be added dropwise, and the reaction can be refluxed for 35 hours to generate succinonitrile.
Refining method: Sublimation and refining succinonitrile. Or use acetone to recrystallize and then perform vacuum distillation to obtain high-purity products.
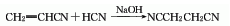
Purpose
1. Mainly used as raw material for quinacridone pigments. This pigment is widely used in automotive and galvanized iron coatings, color printing pigments and colorants for plastic products. Also used in the production of nylon-4 and pharmaceutical intermediates. Can also be used as a reagent. It is used to extract aromatic hydrocarbons from petroleum fractions, and is also used as a polishing agent and organic synthesis raw material in nickel plating.
2. Used in organic synthesis. [25]



 微信扫一扫打赏
微信扫一扫打赏
