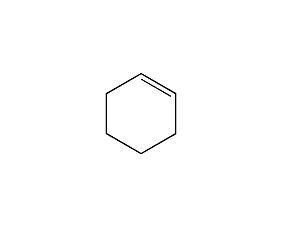
Structural formula
| Business number | 0327 |
|---|---|
| Molecular formula | C6H10 |
| Molecular weight | 82.14 |
| label |
Tetrahydrobenzene, petroleum extractant, High octane gasoline stabilizer, Hydrocarbon solvents |
Numbering system
CAS number:110-83-8
MDL number:MFCD00001539
EINECS number:203-807-8
RTECS number:GW2500000
BRN number:906737
PubChem number:24867583
Physical property data
1. Properties: Colorless and transparent liquid with a special pungent odor. [1]
2. Melting point (℃): -104[2]
3. Boiling point (℃): 83.0[3]
4. Relative density (water = 1): 0.81[4]
5. Relative vapor Density (air=1): 2.8[5]
6. Saturated vapor pressure (kPa): 8.9 (20℃)[6]
7. Heat of combustion (kJ/mol): -3751.7[7]
8. Critical temperature (℃): 287.4[8]
9. Critical pressure (MPa): 4.347[9]
10. Octanol/water partition coefficient: 2.86 [10]
11. Flash point (℃): -11.7[11]
12. Ignition temperature (℃): 244[12]
13. Explosion upper limit (%): 5.0[13]
14. Explosion lower limit (% ): 1.0[14]
15. Solubility: Insoluble in water, miscible in ethanol, ether, acetone, benzene, carbon tetrachloride, petroleum ether, etc. [15]
16. Viscosity (mPa·s, 20ºC): 0.650
17. Flash point (ºC, in oxygen): 325
18. Heat of evaporation (KJ/mol, b.p.): 30.499
19. Heat of fusion (KJ/mol): 40.01
20. Heat of formation (KJ/mol) , 25ºC): -38.22
21. Specific heat capacity (KJ/(kg·K), 25ºC, gas, constant pressure): 1.28
22. Specific heat capacity (KJ/(kg·K) K), 25ºC, liquid, constant pressure): 1.82
23. Conductivity (S/m): 1.5×10-5
24. Thermal conductivity (W/(m·K)): 145.282×10-3
25. Aniline point (ºC): -20.0
26 .Critical density (g·cm-3): 0.287
27. Critical volume (cm3·mol-1): 286
28. Critical compression factor: 0.274
29. Eccentricity factor: 0.214
30. Solubility parameter (J·cm -3)0.5: 17.611
31. van der Waals area (cm2·mol-1): 7.400×109
32. van der Waals volume (cm3·mol-1): 57.040
33. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3785.68
34. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -4.52
35. Gas phase standard entropy (J·mol-1·K-1): 310.63
36. Gas phase standard formation free energy (kJ·mol-1): 107.0
37. Gas phase standard hot melt ( J·mol-1·K-1): 101.5
38. Liquid phase standard heat of combustion (enthalpy) (kJ·mol -1): -3752.21
39. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -37.99
40. Liquid phase standard entropy (J·mol-1·K-1): 216.19
41. Liquid phase%. Note: 1. Since cyclohexene and water can form an azeotrope (azeotropic point 70.8°C, containing 10% water), cyclohexanol and cyclohexene can form an azeotrope (azeotropic point 64.9°C, containing 30.5% cyclohexanol) ), cyclohexanol and water form an azeotrope (azeotropic point 97.8°C, water content 80%), so the temperature is not likely to be too high during heating, and the distillation speed is not likely to be too fast to reduce the loss of cyclohexanol. 2. Cyclohexene can also be synthesized by using phosphoric acid instead of sulfuric acid and adding cyclohexanol dropwise at 165 to 170°C, with a yield of 79% to 84%. [26]
4. Preparation method:
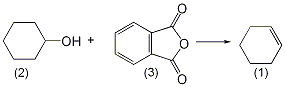
In a reaction flask equipped with a stirrer, dropping funnel, and fractionation device (the receiving flask is cooled with an ice-salt bath), add phthalic anhydride (3) 148g (1.0mol), 5g benzenesulfonic acid. Add 100 mL (1.0 mol) of cyclohexanol (2) to the dropping funnel. First add about 10 mL of cyclohexanol, heat the oil bath to a slight boil while stirring, then slowly add the remaining cyclohexanol dropwise, and finish the addition in about 1 hour. After the addition is completed, continue the reaction for 10 to 15 minutes. Separate the water from the obtained distillate, dry it over anhydrous sodium sulfate, fractionate, and collect the fractions at 82-84°C to obtain 50-64g of cyclohexene, with a yield of 61%-80%. [27]
5. Preparation method:
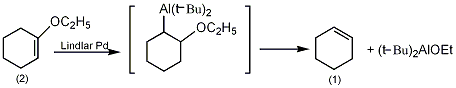
In a reaction flask equipped with a stirrer and distillation device, add 7.21g (0.0572mol) of 1-ethoxycyclohexene (2) and isooctane 30mL. Heat to boiling. Add 16.5g (0.116mol) of diisobutylaluminum hydride in batches within 1.5h, control the addition speed and the instrument, and allow the product to slowly evaporate. After addition, continue heating until approximately 31 mL is evaporated. Re-fractionate and collect the following various fractions: A, 88~94℃, 1.08g, n25D 1.4158; B, 94~99℃, 2.96g, n25 D 1.3985; C, 99~99.5℃, 2.20g, n25D 1.3901. Gas chromatography and infrared spectroscopy proved that each fraction was a mixture of cyclohexene and isooctane. The total amount of cyclohexene was 1.5g, and the yield was 32%. [28]
Purpose
1. Used in organic synthesis, oil extraction and as solvent. It can also be used as catalyst solvent, petroleum extraction agent, and high-octane gasoline stabilizer.
2. Used in organic synthesis, oil extraction and as solvent. [25]



 微信扫一扫打赏
微信扫一扫打赏
