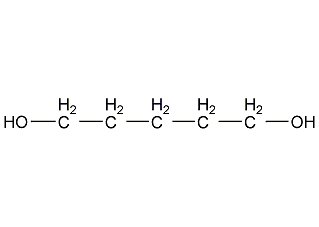
Structural formula
| Business number | 0336 |
|---|---|
| Molecular formula | C5H12O2 |
| Molecular weight | 104.15 |
| label |
Pentane-1,5-diol, Pentamethylene glycol, antifreeze, D, ink solvent, special detergent |
Numbering system
CAS number:111-29-5
MDL number:MFCD00002978
EINECS number:203-854-4
RTECS number:SA0480000
BRN number:1560130
PubChem number:24887075
Physical property data
1. Properties: Colorless, viscous liquid with a bitter taste.
2. Boiling point (ºC, 101.3kPa): 239
3. Boiling point (ºC, 6.67kPa): 160
4. Boiling point (ºC, 1.33 kPa): 134
5. Melting point (ºC): -16
6. Relative density (g/mL, 20/20ºC): 0.994
7 . Relative density (g/mL, 20/4ºC): 0.991
8. Refractive index (n20ºC): 1.4499
9. Viscosity (mPa·s, 20ºC): 128
10. Flash point (ºC, open): 130
11. Fire point (ºC): 335
12. Heat of vaporization (KJ/kg, b.p.) : 606
13. Heat of formation (KJ/mol): 439.9
14. Heat of combustion (KJ/mol): 3158.9
15. Vapor pressure ( kPa, 20ºC): 0.93
16. Lower explosion limit (%, V/V): 1.2
17. Upper explosion limit (%, V/V): 7.7
18. Solubility: miscible with water, low molecular alcohol, and acetone. Insoluble in benzene, methylene chloride and petroleum ether. Dissolves 11% in diethyl ether at 25°C.
19. Refractive index at room temperature (n25): 1.4487
20. Solubility parameter (J·cm-3 )0.5: 26.713
21. van der Waals area (cm2·mol-1): 9.670×10 9
22. van der Waals volume (cm3·mol-1): 67.230
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3233.5
24. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -448.9
25. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -3151.08
26. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -531.45
27. Liquid phase standard entropy (J·mol– 1·K-1): 321.41
28. Liquid phase standard free energy of formation (kJ·mol-1): – 324.05
29. Liquid phase standard hot melt (J·mol-1·K-1): 225.2
Toxicological data
1. Irritation: Rabbit skin open irritation test: 495mg mild irritation.
2. Acute toxicity: Oral LD50 in mice: 6300mg/kg
Oral LD50 in rats: 2000mg/kg
Mouse abdominal LD50: 2 250mg /kg
Rabbit oral LD50: 6300mg/kg
Rabbit transdermal LD50: >20ml/kg
Guinea pig oral LD50: 4600mg/kg
Ecological data
Mildly hazardous to water.
Molecular structure data
1. Molar refractive index: 28.28
2. Molar volume (cm3/mol): 106.0
3. Isotonic specific volume (90.2K ): 264.5
4. Surface tension (dyne/cm): 38.6
5. Polarizability (10-24cm3):11.21
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.1
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 25.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with oxides and moisture.
Storage method
Store sealed in a cool, dry place. Make sure the workplace has good ventilation facilities and is away from sources of fire and water. Store away from oxidizing agents.
Synthesis method
1, using tetrahydrofurfural as raw material, catalytically added Derived from hydrogen. The hydrogenation operation is carried out under high temperature and high pressure, and the reaction temperature is 300-310℃, the pressure is 22-42MPa, industrialization has It must be difficult to first convert tetrahydrofurfuryl alcohol into dihydropyran, then hydrate dihydropyran to produce 5-hydroxyvaleral, and then catalytically hydrogenate it. 1,5-pentanediol is obtained. Add dihydropyran, water and a small amount of hydrochloric acid into the hydration kettle. 50℃ reaction 30-40min, use Neutralize the reaction solution with sodium hydroxide solution and then distill it to remove the water first and then collect60-64℃(0.4kPa) fraction to obtain 5-hydroxyvaleraldehyde, add it to the autoclave, and under the action of nickel catalyst, 120-150℃ , hydrogenation at a pressure of about 6 MPa. After the reaction is completed, distill under reduced pressure to collect 119-120℃ (0.4kPa) fraction is the finished product.
2, epoxypentenal is obtained by photooxidation of cyclopentadiene, and then 70-100℃, obtained by catalytic hydrogenation at about 7MPa pressure.
Purpose
Used as a solvent for cutting oils, special detergents, latex paints, and solvents or wetting agents for inks. Also used in the manufacture of plasticizers, brake oil, alkyd resin, polyurethane resin, etc.
��Special detergents, solvents for latex paints, solvents or wetting agents for inks. Also used in the manufacture of plasticizers, brake oil, alkyd resin, polyurethane resin, etc.



 微信扫一扫打赏
微信扫一扫打赏
