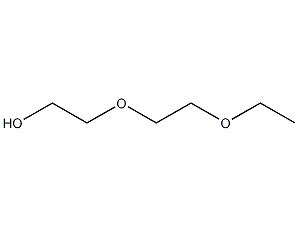
Structural formula
| Business number | 034M |
|---|---|
| Molecular formula | C6H14O3 |
| Molecular weight | 134.17 |
| label |
Diethylene glycol monoethyl ether, Ethoxydiglycol ether, Ethyl carbitol, Diethylene glycol ether, Carbitol, Diethylene glycol monoethyl ether, Ethoxy diethylene glycol ether, car engine detergent, fuel additives, glass cleaner, paint stripper, Polyvinyl acetate emulsifier additive |
Numbering system
CAS number:111-90-0
MDL number:MFCD00002872
EINECS number:203-919-7
RTECS number:KK8750000
BRN number:1736441
PubChem number:24902041
Physical property data
1. Properties: colorless liquid. Slightly pleasant smell, slightly sticky.
2. Density (g/mL, 20/4ºC): 0.9881
3. Density (g/mL, 25/4ºC): 0.9855
4 . Relative vapor density (g/mL, air=1): 4.62
5. Melting point (ºC): -78
6. Boiling point (ºC, normal pressure): 195
7. Refractive index (20ºC): 1.4300
8. Refractive index (25ºC): 1.4273
9. Refractive index (40ºC): 1.4194
10. Viscosity (mPa·s, 20ºC): 3.85
11. Viscosity (mPa·s, 25ºC): 3.71
12. Viscosity (mPa·s, 60ºC): 1.72
13. Flash point (ºC, closed): 94
14. Flash point (ºC, open): 96
15. Evaporation Heat (KJ/mol, b.p.): 47.48
16. Specific heat capacity (KJ/(kg·K), 25ºC, constant pressure): 2.25
17. Conductivity (S/ m, 25ºC): 2.5×10-8
18. Vapor pressure (kPa, 25ºC): 0.017
19. Volume expansion coefficient (K-1, 10~30ºC): 0.00082
20. Solubility: miscible with water, methanol, acetone, ether, carbon tetrachloride, benzene, etc. Soluble 2% in heptane at 25°C. Dissolve grease, resin, dyes, nitrocellulose, polyvinyl acetate, etc. Cellulose acetate does not dissolve.
21. Critical temperature (ºC): 396.85
22. Critical pressure (MPa): 3.167
23. Eccentricity factor: 0.901
24. Liquid phase standard hot melt (J·mol-1·K-1): 307.8
Toxicological data
1. Acute data: LD50: 5540 mg/kg (rat oral); 6580 mg/kg (mouse oral)
2. Irritation data: Skin – rabbit 500 mg/24 Hours Mild; Eyes – Rabbit 125 mg Mild.
3. In case of excessive intake, severe central nervous system depression and respiratory system damage will occur, thirst and acidosis, and albumin will be contained in the urine. It can cause nausea, vomiting, diarrhea, headache, waist and abdominal pain, transient polyuria, and subsequent oliguria, and can cause renal failure.
Ecological data
Slightly harmful to water.
Molecular structure data
1. Molar refractive index: 34.87
2. Molar volume (cm3/mol): 137.8
3. Isotonic specific volume (90.2K ): 326.9
4. Surface tension (dyne/cm): 31.6
5. Polarizability (10-24cm3): 13.82
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 38.7
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 47.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, acid chlorides, and acid anhydrides.
Colorless liquid with aromatic odor. Miscible with water, alcohol, ketone, ether, carbon tetrachloride, aromatic hydrocarbons and aliphatic hydrocarbons. Hygroscopic. This product has low toxicity. It has the chemical properties of alcohol and ether.
2. Has an inhibitory effect on the central nervous system. Rat oral LD508690mg/kg. Avoid prolonged skin contact. Operators should wear respirators to avoid long-term inhalation of large amounts.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. 2. Packed in glass bottles and reinforced with wooden boxes to prevent impact, heat and fire. Store and transport according to regulations for flammable and toxic substances.
Synthesis method
1. Ethylene oxide method: first add absolute ethanol and boron trifluoride to the reactor–Ether solution, then add ethylene oxide. When the reaction temperature rises to 45℃, cool and control the reaction temperature at 70℃Following. After the reaction is completed, neutralize and distill under reduced pressure to collect 90℃(0.1MPa) or less, the finished product is obtained.
2.DiethyleneglycolmethodconsistsoftwoProducedbythereactionofglycolanddiethylsulfate.
3.In the industrial production of ethylene glycol monoethyl ether, the generated ethylene glycol can be Monoethyl ether continues to react with ethylene oxide to obtain the product diethylene glycol monoethyl ether.

Refining method: generally contains 30% ethylene glycol, ethylene glycol monoethyl ether, triethylene glycol monoethyl ether and a small amount of water and acid. It is difficult to remove ethylene glycol by fractional distillation alone, and a solvent separation method must be used at the same time. For this purpose, add 750mL benzene and 5mL water to the 250g sample, shake it and let it stand for 10 minutes, and accurately measure the volume of the water layer. The volume initially increases, and the operation is repeated several times until the volume no longer increases. After further fractionation, pure diglycol monoethyl ether can be obtained.
Purpose
1. This product can dissolve oils, fats, paraffin, dyes, camphor and resins, cowrie rosin, frankincense rosin, sandalwood, shellac and other natural resins and several kinds of synthetic resins, and can be used in cellulose esters, paints The solvent can also be used as a diluting composition, quick drying brightener and colorant for enamel products and wood stain. Used as a solvent for nitrocellulose, resins, printing inks, dyes for wood coloring, etc. It is also used as car engine detergent, fuel additive, glass cleaner, paint stripper, polyvinyl acetate emulsifier additive, etc. In addition, it is also used for the separation of propyne from acetylene and the manufacture of textile soap, cortisone acetate, etc.
2. High boiling point solvent, capable of dissolving oils, fats, paraffin, dyes, camphor, and silica rosin, frankincense, sandalwood, and shellac It has the functions of natural resin and synthetic resin, and can be used as a base component of synthetic resin and a paint solvent. It is also a solvent and thinner for nitrocellulose paints and other synthetic dyes, baking varnishes, quick-drying printing inks. It can also be used as a mutual solvent and fiber dyeing agent for mineral oil soap and mineral oil vulcanized oil mixtures, as well as a raw material for making nail polish.
3.Because it has both hydroxyl and ether groups, it has excellent solubility and is the most typical industrial solvent. In the detergent industry, it is used as automobile engine cleaner, glass cleaner, paint remover, stain remover, etc. It is also used as a solvent for cellulose, resin, paint, spray paint, and ink.
, can also be used as a dilute composition, quick drying brightener and colorant for enamel products and wood stain. Used as a solvent for nitrocellulose, resins, printing inks, dyes for wood coloring, etc. It is also used as car engine detergent, fuel additive, glass cleaner, paint stripper, polyvinyl acetate emulsifier additive, etc. In addition, it is also used for the separation of propyne from acetylene and the manufacture of textile soap, cortisone acetate, etc.
2. High boiling point solvent, capable of dissolving oils, fats, paraffin, dyes, camphor, and silica rosin, frankincense, sandalwood, and shellac It has the functions of natural resin and synthetic resin, and can be used as a base component of synthetic resin and a paint solvent. It is also a solvent and thinner for nitrocellulose paints and other synthetic dyes, baking varnishes, quick-drying printing inks. It can also be used as a mutual solvent and fiber dyeing agent for mineral oil soap and mineral oil vulcanized oil mixtures, as well as a raw material for making nail polish.
3.Because it has both hydroxyl and ether groups, it has excellent solubility and is the most typical industrial solvent. In the detergent industry, it is used as automobile engine cleaner, glass cleaner, paint remover, stain remover, etc. It is also used as a solvent for cellulose, resin, paint, spray paint, and ink.



 微信扫一扫打赏
微信扫一扫打赏
