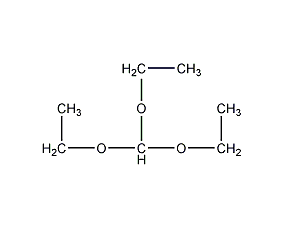
Structural formula
| Business number | 03F3 |
|---|---|
| Molecular formula | C7H16O3 |
| Molecular weight | 148 |
| label |
Triethoxymethane; Ethyl orthoformate, Aliphatic carboxylic acids and their derivatives |
Numbering system
CAS number:122-51-0
MDL number:MFCD00009230
EINECS number:204-550-4
RTECS number:RM6475000
BRN number:605384
PubChem number:24886840
Physical property data
1. Properties: colorless and transparent liquid with spicy smell. [9]
2. Melting point (℃): -61[10]
3. Boiling point (℃): 145.9[11]
4. Relative density (water = 1): 0.89[12]
5. Relative vapor Density (air=1): 5.11[13]
6. Saturated vapor pressure (kPa): 1.33 (40.5℃)[14]
7. Octanol/water partition coefficient: 1.2[15]
8. Flash point (℃): 30 (CC); 35 (OC) [16]
9. Ignition temperature (℃): 180[17]
10. Explosion limit (% ): 25.1[18]
11. Lower explosion limit (%): 0.7[19]
12. Solubility : Slightly soluble in water, soluble in most organic solvents such as ethanol and ether. [20]
13. Refractive index at room temperature (n25): 1.3900
14. Gas-phase standard combustion heat (enthalpy )(kJ·mol-1): -4405.9
15. Gas phase standard claims heat (enthalpy) (kJ·mol-1): – 635.3
16. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -4359.90
17. Liquid phase standard claimed heat ( Enthalpy) (kJ·mol-1): -681.32
Toxicological data
1. Acute toxicity[21] LD50: 7060mg/kg (rat oral); 20ml (17800mg)/kg (rabbit transdermal)
2. Irritation [22]
Rabbit transdermal: 500mg (24h), mild irritation.
Rabbit eye: 100mg (24h), moderate irritation.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 39.67
2. Molar volume (cm3/mol): 163.5
3. Isotonic specific volume (90.2K ): 367.0
4. Surface tension (dyne/cm): 25.3
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 15.72
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 51.6
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain atomic stereocenterNumber of stereocenters: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is very sensitive to moisture, flammable, has strong volatility and pungent odor, and should be used in a fume hood.
2. Stability[23] Stable
3. Incompatible substances[24] Strong oxidants, water, strong acids
4. Conditions to avoid contact[25] Humid air
5. Hazards of aggregation[26] No aggregation
Storage method
Storage Precautions[27] Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, acids, etc., and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Sodium ethoxide method: Using sodium ethoxide and chloroform as raw materials, react at 60-65°C to generate crude triethyl orthoformate, which is then refined by fractionation to obtain the finished product.
![]()
2. Ethanol method: use ethanol and sodium hydroxide instead of sodium ethoxide, using solid-liquid phase transfer catalysis method, and the catalyst is PTC.
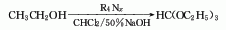
3. Benzoyl chloride method: Using benzoyl chloride, formamide and absolute ethanol as raw materials, under the action of a catalyst, a mixture of triethyl orthoformate, benzoic acid and ammonium chloride is generated, and the finished products triethyl orthoformate and benzoic acid are obtained after separation.
4. Place 3 liters of absolute ethanol and 490 grams (327 ml, 4.1 mol) of chloroform in a 5-liter round-bottom flask equipped with an 80-cm-long reflux condenser. The flask should be placed Easy to use running water cooling location. 207 grams (9 grams atoms) of clean sodium metal were cut into small pieces so that they would fit through the condenser and then added to the solution over about two hours. In order to add metallic sodium at this rate, the flask must be cooled while adding sodium. When the metallic sodium has completely acted and the mixture has cooled to room temperature, use a drying instrument to filter out the sodium chloride by suction filtration, wash the sodium oxide on the filter paper with 200 ml of absolute ethanol, and let the washing liquid flow into the filtrate obtained above. middle.
Put the filtrate into a 3-liter flask equipped with an 80 cm fractionating column, and evaporate excess chloroform and most of the ethanol on a steam bath or water bath. Collect the distillate in a 2-liter suction filter bottle, which is equipped with a drying flute to isolate moisture. Distillation takes 5 to 6 hours. The mixture of chloroform and ethanol recovered in the distillation towel weighs about 2000 grams and can be kept for use in the next experiment. The remaining liquid in the flask is removed by decantation to remove a small amount of sodium chloride, poured into a Kirschner flask with a 30 cm radium column, and distilled under normal pressure. The fraction below 85°C is mainly ethanol and will be discarded; the fraction between 85-140°C is about 100 grams, which contains about a quarter of the total output of ethyl formate. This fraction can be fractionated, but it is better to combine it with the product of the next experiment. Original methamphetamine vinegar is distilled at 140-146°C and weighs 120-140 grams (27-31%).
After the first experiment, add 400 grams of chloroform phase and enough anhydrous ethanol (800-1000 ml) to the recovered chloroform-ethanol mixture to bring the total amount to 3 liters. Then add metallic sodium to the previous rod. After the excess chloroform and ethanol have been distilled from the distillation column, add the middle portion of the previous experiment and proceed with the fractional distillation. The yield of this experiment was about 200 grams 45%.
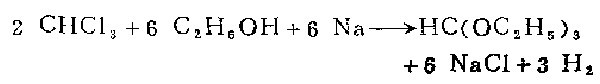
Purpose
1. Triethyl orthoformate is a commonly used alkylating reagent and formylating reagent in chemical laboratories. They are easy to store but highly reactive. They can be used as alkylating reagents and can easily transfer alkyl groups to various alcohol hydroxyl groups; when used as formylating reagents, they can be used under acidic or alkaline conditions.
Alkylation reaction with alcohol When triethyl orthoformate is used as an alkylation reagent, it can cause corresponding alkylation reactions of various alcohols. Alkylation reaction of polyols can occur to generate the corresponding cyclic orthoformate (Formula 1)[1~3].
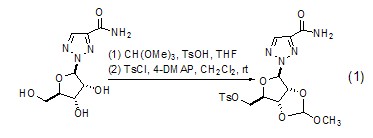
Aldehyde or ketone Protection Orthoformate can be used to protect aldehydes and ketones to generate formate and the corresponding acetal or ketal (formula 2)[4,5].

Formylation reaction Under the catalysis of Lewis acid, orthoformate can be used as a formylation reagent. Compared with other formylation reagents, it has the characteristics of easy operation and higher yield. Under acid catalysis, orthoformate can easily generate dialkoxy carbonium ions in situ and can be directly used in the formylation reaction. This type of formylation reaction can be on the α-C ortho to the carbonyl group (Formula 3)[6].
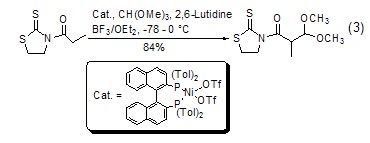
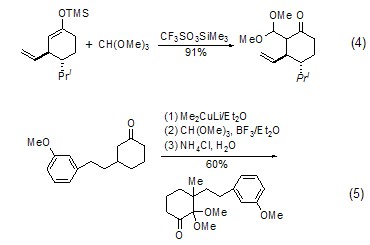
With enol ( or analogues) reaction The silyl ether of enol (formula 4)[7] or the enolate of ketone (formula 5) [8] can be reacted with The carbonium ion generated in situ by orthoformate reacts to generateβ-acetal ketone compounds.
2. Used for organic synthesis and Used as pharmaceutical intermediates and photosensitive materials. [28]

With enol ( or analogues) reaction The silyl ether of enol (formula 4)[7] or the enolate of ketone (formula 5) [8] can be reacted with The carbonium ion generated in situ by orthoformate reacts to generateβ-acetal ketone compounds.

2. Used for organic synthesis and Used as pharmaceutical intermediates and photosensitive materials. [28]



 微信扫一扫打赏
微信扫一扫打赏
