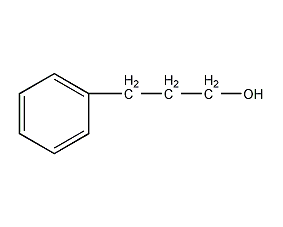
Structural formula
| Business number | 03FU |
|---|---|
| Molecular formula | C9H12O |
| Molecular weight | 136.19 |
| label |
3-phenylpropanol, cholerol, Hydrogenated cinnamyl alcohol, 3-Phenylpropyl alcohol, Hydrocinnamyl alcohol, C6H5(CH2)3OH, food additives, Flavor enhancer |
Numbering system
CAS number:122-97-4
MDL number:MFCD00002950
EINECS number:204-587-6
RTECS number:UB8970000
BRN number:1857542
PubChem number:24901373
Physical property data
1. Properties: colorless liquid
2. Boiling point: 237.5750℃, 119℃ (1.6kPa)
3. Relative density: 0.995 (25/4℃)
4. Refractive index: 1.5357 (25℃)
5. Flash point: 109℃
6. Solubility: soluble In 70% ethanol and ether, slightly soluble in water
7. Relative density (25℃, 4℃): 0.9977
8. Refractive index at room temperature (n20): 1.5271
9. Refractive index at room temperature (n25): 1.5245
10. Relative density (20℃, 4℃): 1.001778
Toxicological data
1. Skin/eye irritation: Rabbit skin standard Drez eye dye test: 10mg/24H is moderately irritating to the skin.
2. Acute toxicity: Rat oral LD50: 2300mg/kg
Rabbit skin LD50: 5gm/kg
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 41.96
2. Molar volume (cm3/mol): 136.2
3. Isotonic specific volume (90.2K): 340.3
4. Surface tension (dyne/cm): 38.9
5. Polarizability (10-24cm3): 16.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 74.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain chemical bondsNumber of stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Properties: colorless liquid.
Storage method
None yet
Synthesis method
1. Preparation method: prepared by catalytic hydrogenation of ethyl cinnamate. The hydrogenation reaction is carried out in an autoclave, using a chromium-copper-barium catalyst, the temperature is 200°C, and the hydrogen pressure is about 20MPa. After 5-9 hours of hydrogenation reaction, the catalyst was filtered off by cooling, and the filtrate was extracted with diethyl ether. After recovering ether from the extract, perform vacuum distillation and collect the 110-112°C (1.06kPa) fraction, which is the finished product, with a yield of about 85%. Another preparation method is to obtain 3-phenylpropanol magnesium chloride salt through Grignard reaction between benzyl chloride and ethylene oxide, and then hydrolyze it with sulfuric acid to obtain 3-phenylpropanol. The yield of this method is about 65-70%.
2. Preparation method:
![]()
Add 57g (0.32mol) of ethyl cinnamate (2) and 5g of chromium-copper-barium catalyst into the high-pressure reaction kettle, and seal the reaction kettle. Replace the air with nitrogen, and then replace the nitrogen with hydrogen. Pour in hydrogen to a pressure of 20MPa until hydrogen is no longer absorbed, which takes about 5 to 9 hours. After the reaction is completed, the remaining pressure is released, the reaction kettle is opened, the liquid is taken out, the catalyst is filtered off, and washed with diethyl ether. After distilling off the diethyl ether, perform fractional distillation under reduced pressure and collect the fraction at 110-112°C/1.06kPa to obtain 27g of 3-phenyl-1-benzyl alcohol (1) with a yield of 85%. [1]
3. Preparation method:
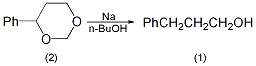
Equipped with a stirrer and two reflux condensers In the 5L reaction bottle, add 925mL of dry toluene and 168g of metallic sodium (7.3mol). Heat the oil bath to boiling and start stirring after the metallic sodium melts. Remove the heat source, and add dropwise a solution of 328g (2.0mol) of 4-phenyl-m-dioxane (2) dissolved in 311g (4.2mol) of n-butanol from the top of a condenser, and complete the addition in about 30 to 60 minutes. Cool to room temperature, and slowly add dropwise a solution of 100 mL concentrated sulfuric acid dissolved in 800 mL water. Separate the organic layer, add 500 mL of water and 5% sulfuric acid to the organic layer to make it neutral. Separate the organic layer, recover toluene, distill under reduced pressure, collect the fraction at 113~115℃/0.4kPa, and obtain 224~227g of 3-phenyl-1-propanol (1), with a yield of 82.2%~83.4%. [2]
Purpose
3. Use: used in the synthesis of spices and drugs. In the pharmaceutical industry, it is an intermediate of the central skeletal muscle relaxant prednisone. This product has a sweet floral aroma and sweet preserves aroma, and after dilution it has a fresh and pleasant melon and fruit aroma. Natural products exist in strawberries, fungus balm, benzoin balm, tea, cinnamon leaf oil, etc., and are temporarily allowed to be used as edible spices in my country’s GB2760-86.



 微信扫一扫打赏
微信扫一扫打赏
