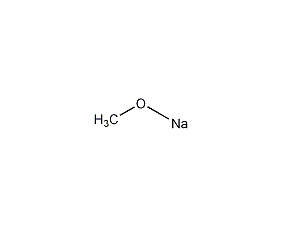
Structural formula
| Business number | 03J6 |
|---|---|
| Molecular formula | CH3ONa |
| Molecular weight | 54.02 |
| label |
Methanol sodium salt, sodium methoxide, Sodium methoxide methanol solution, Sodium methoxide solution, Methanolic Base, food processing aids, food additives |
Numbering system
CAS number:124-41-4
MDL number:MFCD00012179
EINECS number:None
RTECS number:None
BRN number:3592982
PubChem ID:None
Physical property data
1. Properties: white powder, sensitive to air and moisture. Odorless
2. Boiling point: >450℃
3. Solubility: soluble in methanol and ethanol. water.
Toxicological data
Acute toxicity data:
Rat oral LD50: 2037 mg/kg is toxic and highly corrosive
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.1
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 4.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
1. Basic properties: Easily decomposes when exposed to light and moisture in the air. It decomposes into methanol and sodium hydroxide when meeting water. It reacts with methanol to form CH3ONa·2CH3OH (white powder).
2. The methanol in sodium methoxide solution is highly toxic. Sodium methoxide is highly corrosive and can irritate the eyes and nose. Inhaling high-concentration vapor can cause strong irritation. In severe cases, it can affect breathing and cause dizziness, which can be recovered with rescue. However, inhaling high-concentration vapor for a long time can cause pulmonary edema and death. Inhalation of vapors and contact with skin should be prevented. In addition, safety measures should be paid attention to during the production process, equipment should be sealed, and operators should wear protective equipment.
Storage method
1. Storage: sealed and stored in a cool, dry and dark place
2. Sealed and packed in iron drums, 200kg per drum, stored in a cool, ventilated and dry place, fireproof and heat-proof ,Protect
from the sun. Store and transport according to regulations on flammable chemicals.
Synthesis method
Brief description of the production method: The industrial product is usually a methanol solution of sodium methoxide (the content is generally 23%).�� is obtained by reacting methanol with sodium hydroxide, and adding benzene at the same time for azeotropic distillation, so that benzene, methanol, and water form a ternary azeotrope. After removing the water generated during the reaction, the benzene is evaporated.
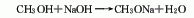
Purpose
1.Uses: Mainly used as raw materials for medicines and pesticides, and also used in dye and chemical fiber industries. Organic synthesis
2. Mainly used as condensation agent and strong alkaline catalyst, and methoxylating agent for producing vitamin B1 and A, Sulfadiazine and other drugs. A small amount is used in pesticide production. It can also be used as a catalyst for processing edible fats and oils (especially lard). It can also be used as an analytical reagent.



 微信扫一扫打赏
微信扫一扫打赏
