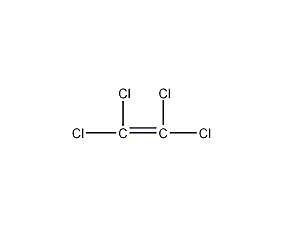
Structural formula
| Business number | 03KP |
|---|---|
| Molecular formula | C2Cl4 |
| Molecular weight | 165.83 |
| label |
perchlorethylene, Perchloroethylene, Tetrachloroethene, Extracting agent, fiber dry cleaning agent, Degreasing detergent for metals, Desiccant, paint stripper, insect repellent, Aliphatic halogenated derivatives |
Numbering system
CAS number:127-18-4
MDL number:MFCD00000834
EINECS number:204-825-9
RTECS number:KX3850000
BRN number:1361721
PubChem number:24863125
Physical property data
1. Properties: colorless liquid with chloroform-like odor. [1]
2. Melting point (℃): -22.3[2]
3. Boiling point (℃): 121.2[3]
4. Relative density (water = 1): 1.63[4]
5. Relative vapor Density (air=1): 5.83[5]
6. Saturated vapor pressure (kPa): 2.11 (20℃)[6]
7. Heat of combustion (kJ/mol): -679.3[7]
8. Critical temperature (℃): 347.1[8]
9. Critical pressure (MPa): 9.74[9]
10. Octanol/water partition coefficient: 2.6~3.4[10]
11. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, and chloroform. [11]
12. Vapor pressure (kPa, -20.6ºC): 0.13
13. Vapor pressure (kPa, 2.4ºC): 0.67
14. Vapor pressure (kPa, 13.8ºC): 1.33
15. Vapor pressure (kPa, 121.2ºC): 101.33
16. Viscosity (mPa· s, 20ºC): 0.880
17. Heat of evaporation (KJ/mol, b.p.): 34.75
18. Heat of formation (KJ/mol, gas): -25.12
19. Heat of formation (KJ/mol, liquid): 12.56
20. Heat of fusion (KJ/mol): 10.57
21. Heat of combustion (KJ/mol , 25ºC, liquid): 680.4
22. Specific heat capacity (KJ/(kg·K), 20ºC, constant pressure): 0.904
23. Electrical conductivity (S/m, 20ºC ): 5.55×10-4
24. Volume expansion coefficient (K-1, 0~25ºC): 0.00102
25. Volume expansion coefficient (K-1, 15~95ºC): 0.001079
26. Refractive index at room temperature (n20): 1.5053
27. Refractive index at room temperature (n25): 1.5028
28. Solubility parameter (J·cm-3) 0.5: 18.956
29. van der Waals area (cm2·mol-1): 8.500×109
30. van der Waals volume (cm3·mol-1): 58.980
31. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -51.8
32. Liquid phase standard hot melt (J·mol– 1·K-1): 147.0
33. Gas phase standard claims heat (enthalpy) (kJ·mol-1): -12.1
34. Gas phase standard entropy (J·mol-1·K-1): 340.96
35. Gas phase standard free energy of formation (kJ·mol-1): 22.7��Trichloroacetyl chloride and a small amount of tetrachlorodinitroethane. It does not react when heated with concentrated nitric acid, but reacts with fuming nitric acid to produce trichloroacetyl chloride and tetrachlorodinitroethane. Reacts with nitrogen dioxide at 100°C to form tetrachlorodinitroethane. Tetrachloroethane is produced during hydrogenation. It reacts with ammonia under high pressure and decomposes into ammonium chloride and carbon. It reacts explosively with potassium metal near its melting point, but does not react with sodium metal. After photochlorination reaction, hexachloroethane is generated. It reacts with a mixture of hydrogen fluoride and chlorine under the catalysis of zirconium fluoride at 225~400℃ to obtain 1,2,2-trichloro-1,1,2-trifluoroethane CClF2CCl2F (Freon-113). In the presence of aluminum trichloride, it undergoes condensation reaction with other chlorinated hydrocarbons to form high boiling point substances.
Storage method
Storage Precautions[23] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging must be sealed and must not come into contact with air. They should be stored separately from alkalis, active metal powders, alkali metals, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Ethylene method This method can co-produce trichlorethylene and tetrachlorethylene and is divided into the following two methods. a) Direct chlorination of ethylene and chlorine in a 1,2-dichloroethane solution containing FeCl3 catalyst at 280-450°C to generate 1,2-dichloroethane, which is further chlorinated into trichloroethylene and After distillation, tetrachlorethylene is neutralized with NH3, washed and dried to obtain the finished product. b) Oxychlorination method uses the addition of ethylene and chlorine to generate 1,2-dichloroethane. 1,2-dichloroethane, chlorine and oxygen react with CuCl2 and KCl as catalysts and under the conditions of 425℃ and 138-207kPa. Carry out oxychlorination reaction, and the product is cooled, washed, dried and then distilled to obtain a high-purity product.
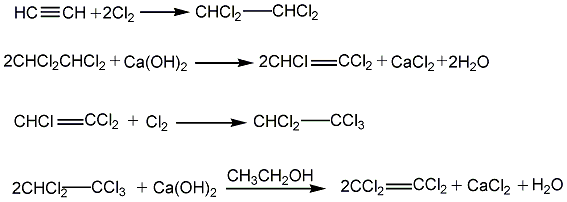
2. Hydrocarbon oxidation method will contain methane, ethyl Hydrocarbon mixtures such as alkane, propane, propylene, etc. are chlorinated and pyrolyzed at 50-500°C to obtain a mixture of chlorinated hydrocarbons, which are separated into various products after distillation.
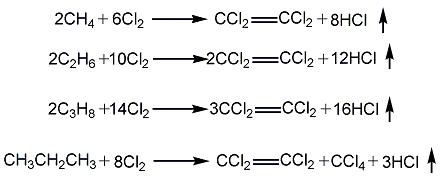
3. Acetylene method Acetylene and chlorine Heating and chlorination generates 1,1,2,2-tetrachloroethane, which is then used to remove hydrogen chloride with alkali to obtain trichloroethylene, which is then chlorinated to generate pentachloroethane, and then used to remove hydrogen chloride with alkali to obtain tetrachloroethylene. Due to the high price of acetylene, it has been gradually replaced by the ethylene method.
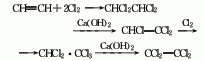
4. Preparation method Acetylene chlorination Afterwards, tetrachloroethane is generated, and the HCl is removed by alkali to become trichlorethylene. It is then chlorinated to become pentachloroethane, and then calcium hydroxide is used to remove HCl to become tetrachlorethylene.
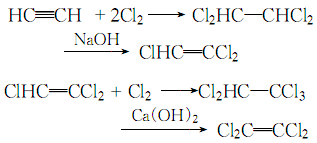
To prepare chromatographically pure tetrachlorethylene , you can use nitrogen as the carrier gas, inject the crude product on a preparative gas chromatograph equipped with a SE30/white diatomite carrier stationary phase column, collect the main peak components after separation, and then put it into a glass ampoule and seal it.
5. Obtained from the dehydrogenation of pentachloroethane and lime milk.
Purpose
1. Tetrachlorethylene is widely used, mainly used as organic solvents, dry cleaning agents, metal degreasing solvents, and also as anthelmintics. Tetrachlorethylene can be used as a fat extraction agent, fire extinguishing agent and smoke screen agent, etc. It can also be used to synthesize trichlorethylene and fluorine-containing organic compounds.
2. Widely used as a dry cleaning agent for natural and synthetic fibers. It is also used as a metal degreasing detergent, desiccant, paint remover, insect repellent and general solvent, organic synthesis intermediate, etc.
3. Used as solvent, chromatographic analysis standard material, dry cleaning agent, and also used in organic synthesis.
4. Mainly used as organic solvents and dry cleaning agents. Used as solvents for adhesives, degreasing solvents for metals, desiccants, paint strippers, insect repellents, fat extractants, and also used in organic synthesis.
5. Used as solvent. [24]



 微信扫一扫打赏
微信扫一扫打赏
