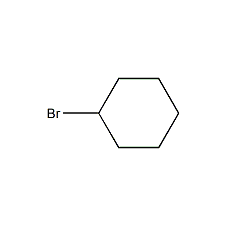
Structural formula
| Business number | 02Y2 |
|---|---|
| Molecular formula | 6H11Br |
| Molecular weight | 163.06 |
| label |
cyclohexyl bromide, bromocyclohexane, Bromocyclohexane, Hexahydrobenzene, Bromocyclohexane, Hexahydrobromobenzene, Bromocyclohexane, Cyelohexyl bromide, Cyclohexyl Bromide, 1-Bromocyclohexane, Bromo-cyclohexan, Bromocylohexane, Cyclohexane,bromo-, Cyclohexylbromid |
Numbering system
CAS number:108-85-0
MDL number:MFCD00003819
EINECS number:203-622-2
RTECS number:GU7171700
BRN number:1098373
PubChem number:24886833
Physical property data
1. Properties: colorless liquid. Has a pungent odor. Sensitive to light.
2. Density (g/mL, 25/4℃): 1.258385.7
3. Relative density (20℃, 4℃): 1.3359
4. Melting point (ºC): -56.5
5. Boiling point (ºC, normal pressure): 166.2
6. Refractive index at room temperature (n20): 1.4957
7. Refractive index: 1.4956
8. Flash point (ºC): 63
9. Refractive index at room temperature (n25): 1.4935
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC) : Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14 . Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19 . Solubility: Soluble in ethanol and ether, insoluble in water.
Toxicological data
None
Ecological data
1. Other toxic effects: This substance is harmful to the environment. Special attention should be paid to the pollution of water and air. It has extremely destructive power to the atmospheric ozone layer.
Molecular structure data
1. Molar refractive index: 35.45
2. Molar volume (cm3/mol): 120.8
3. Isotonic specific volume (90.2K ): 291.5
4. Surface tension (dyne/cm): 33.8
5. Dielectric constant: undetermined
6. Dipole moment (10 -24cm3):
7. Polarizability: 14.05
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 46.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the reaction of cycloethanol and hydrobromic acid: Heat cycloethanol and 48% hydrobromic acid to reflux for 6 hours. A small amount of water was added to the reaction mixture, and the lower organic phase was separated and dried over anhydrous calcium chloride. Distill and collect the 163-165°C fraction as the finished product. The yield is 74%.
2. Preparation method:
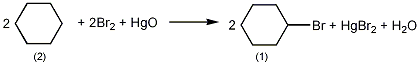
In a reaction bottle equipped with a magnetic stirrer and a reflux condenser, add 2.0g of mercury oxide and 4mL of cyclohexane (2), and stir at room temperature. Then add 2.0 mL (2.02 mol/L) of bromine solution in carbon tetrachloride, and continue stirring the reaction for 20 minutes. During this time the color of bromine gradually disappeared. Analysis proved that 1.58 mmol of bromocyclohexane (1) was produced, with a yield of 79%. Bromocyclohexane is synthesized from cyclohexanol, potassium bromide and sulfuric acid as raw materials. When the molar ratio of potassium bromide to cyclohexanol is 1:0.8, the yield is about 90%. [1]
Purpose
Used as an intermediate in organic synthesis.



 微信扫一扫打赏
微信扫一扫打赏
