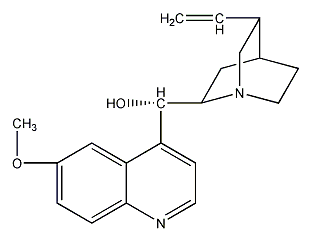
Structural formula
| Business number | 03MP |
|---|---|
| Molecular formula | C20H24N2O2 |
| Molecular weight | 324.42 |
| label |
quinine, Quinine anhydrous, Cinchona base, cinchona, (6-methoxy-4-quinolyl)(5-vinyl-1-azabicyclo[2.2.2]octan-2-yl)methanol, antibiotic, alkaloids |
Numbering system
CAS number:130-95-0
MDL number:MFCD00198096
EINECS number:205-003-2
RTECS number:VA6020000
BRN number:91867
PubChem number:24848751
Physical property data
1. Characteristics: Quinine crystallized in benzene is needle-like crystal, containing solvent. It slowly loses the solvent in the air and turns into tiny crystalline anhydrous matter.
2. Melting point (℃): 173~177
3. Solubility: Hardly soluble in water, easily soluble in ethanol, chloroform and boiling benzene, soluble in anhydrous ether (1:250).
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 95.78
2. Molar volume (cm3/mol): 266.3
3. Isotonic specific volume (90.2K ): 728.7
4. Surface tension (dyne/cm): 56.0
5. Polarizability (10-24cm3): 37.97
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.9
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 4
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 45.6
7. Number of heavy atoms: 24
8. Surface charge: 0
9. Complexity: 457
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 4
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Quinine crystallized in benzene is a needle-shaped crystal containing a solvent. It slowly loses the solvent in the air and becomes a tiny crystalline anhydrous substance.
Storage method
Brown glass bottle with light-sealed packaging. Store in low temperature and dry place.
Synthesis method
1.���The bark or root bark of the cinchona tree is cleaned of impurities, dried, crushed and evenly mixed with soda lime. Then repeatedly extract with petroleum ether, combine the extracts, add dilute sulfuric acid for extraction after clarification, and obtain quinine sulfate after the acid layer is concentrated and crystallized. The finished products are available in two forms: acidic quinine sulfate and neutral quinine sulfate. Acidic quinine sulfate is a salt formed by one molecule of sulfuric acid and one molecule of quinine. It has good water solubility and the aqueous solution is acidic. Neutral quinine sulfate is a salt formed by two molecules of quinine and one part of sulfuric acid. Its aqueous solution is neutral but has poor water solubility. Commonly used in medicine is acidic quinine sulfate.
Purpose
1. Quinine can inhibit the reproduction or kill the intraerythrocytic protozoa of falciparum malaria and is an important antimalarial drug. Quinine also inhibits myocardial contractility and increases uterine rhythmic contractions.



 微信扫一扫打赏
微信扫一扫打赏
