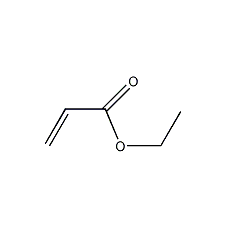
Structural formula
| Business number | 03TE |
|---|---|
| Molecular formula | C5H8O2 |
| Molecular weight | 100.12 |
| label |
Ethyl benanate, Acrylic acid ethyl ester, spices |
Numbering system
CAS number:140-88-5
MDL number:MFCD00009188
EINECS number:205-438-8
RTECS number:AT0700000
BRN number:773866
PubChem number:24886942
Physical property data
1. Properties: colorless liquid with pungent odor. [1]
2. Melting point (℃): -71.2[2]
3. Boiling point (℃): 99.4[3]
4. Relative density (water = 1): 0.92[4]
5. Relative vapor Density (air=1): 3.45[5]
6. Saturated vapor pressure (kPa): 3.90 (20℃)[6]
7. Heat of combustion (KJ/mol): -2739.9[7]
8. Critical temperature (℃): 279[8]
9. Critical pressure (MPa): 3.7[9]
10. Octanol/water partition coefficient: 1.32 [10]
11. Flash point (℃): 9[11]
12. Ignition temperature (℃): 345 ~383[12]
13. Explosion upper limit (%): 14.0[13]
14. Explosion lower limit ( %): 1.4[14]
15. Solubility: Slightly soluble in water, easily soluble in ether, ethanol, and soluble in chloroform. [15]
16. Viscosity (mPa·s, 20ºC): 0.69
17. Viscosity (mPa·s, 25ºC): 0.55
18. Viscosity (mPa·s, 40ºC): 0.50
19. Flash point (ºC, open): 19
20. Flash point (ºC, closed ): 9
21. Vapor pressure (kPa, 0ºC): 1.2
22. Vapor pressure (kPa, 20ºC): 3.93
23. Vapor pressure (kPa, 50ºC): 17.3
24. Relative density (20℃, 4℃): 0.9234
25. Refractive index at room temperature (n25) : 1.4032
26. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -2756.59
27. Gas phase standard claimed heat (enthalpy) )(kJ·mol-1): -354.22
28. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1): -2717.51
29. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -393.30
30. Liquid phase standard hot melt (J·mol-1·K-1):186.7
Toxicological data
1. Acute toxicity[16]
LD50: 760mg/kg (rat oral); 1800mg/kg (mouse oral Oral); 280mg/kg (rabbit oral); 1800mg/kg (rabbit transdermal)
LC50: 1000ppm (rat inhalation, 4h)
2. Stimulation Sex [17]
Rabbit transdermal: 10mg (24h), mild stimulation.
Rabbit eye: 45mg, mild irritation.
3. Subacute and chronic toxicity[18] Rat inhaled 2.209g/m3, died in 19 days The rate is 12/18.
Mutagenicity[19] Micronucleus test: mouse abdominal cavity 225mg/kg. Sister chromatid exchange: Hamster ovary 150mg/L. Cytogenetic analysis: Rat oral administration: 0.5 ppb.
4. Carcinogenicity[20] Acid and ethanol undergo an esterification reaction continuously at the boiling point temperature in the presence of an ion exchange resin catalyst to obtain ethyl acrylate.
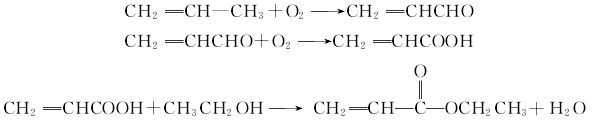
Purpose
1. Mainly used as a comonomer for synthetic resins. The copolymers formed are widely used in coatings, textiles, leather, adhesives and other industries. Ethyl acrylate is an intermediate for the preparation of carbamate insecticide propylcarbofuran. It can also be used as a raw material for protective coatings, adhesives and paper impregnants. Its polymer can be used as an anti-cracking agent for leather. The copolymer with ethylene is a hot-melt adhesive. The copolymer with 5% chloroethyl vinyl ether is a synthetic rubber with good oil resistance and heat resistance. In some cases, it can replace nitrile rubber. .
2. Edible spices allowed to be used in GB 2760-1996. Mainly used for preparing rum, pineapple and assorted fruit flavors.
3. Polymer synthetic material monomer. It is also used in the manufacture of coatings, adhesives, leather processing agents, textile auxiliaries, paint additives, etc. The copolymer with ethylene is a hot melt adhesive; the copolymer with 5% chloroethyl vinyl ether is a synthetic rubber with good oil resistance and heat resistance, which can replace nitrile rubber in some cases.
4. Monomers for manufacturing solvent-based and emulsion-based acrylic adhesives. It can be copolymerized with vinyl acetate, acrylic acid, styrene, etc., and the polymer is used as a raw material for adhesives, coatings, rubber, and synthetic fibers. It can also be used to manufacture leather processing agents, textile auxiliaries and sizing agents, paper impregnating agents, etc. In the food industry, it is used to prepare fruit flavors such as pineapple and rum flavors.
5. Used as an intermediate in organic synthesis and in the preparation of polymers. [29]



 微信扫一扫打赏
微信扫一扫打赏
