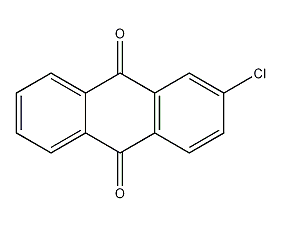
Structural formula
| Business number | 03MR |
|---|---|
| Molecular formula | C14H7ClO2 |
| Molecular weight | 242.66 |
| label |
2-Chloro-9,10-anthracenedione, β-Chloroanthraquinone, β-Chloroanthraquinone, aromatic compounds |
Numbering system
CAS number:131-09-9
MDL number:MFCD00001227
EINECS number:205-010-0
RTECS number:CB6151000
BRN number:2051842
PubChem number:24849604
Physical property data
1. Properties: light yellow needle-like crystals
2. Melting point (℃): 208~211
3. Solubility: soluble in alcohol, ether, hot benzene, Nitrobenzene, concentrated sulfuric acid, ethyl acetate, insoluble in water.
Toxicological data
1. Acute toxicity: Rat oral LD: >10gm/kg
Rat abdominal LD5O: 4310mg/kg
Mouse oral LD: >10gm/ kg
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 63.56
2. Molar volume (cm3/mol): 171.0
3. Isotonic specific volume (90.2K ): 471.9
4. Surface tension (dyne/cm): 58.0
5. Polarizability (10-24cm3):25.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 34.1
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 352
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Low toxicity. Phthalic anhydride and chlorobenzene used in production are toxic, and fuming concentrated sulfuric acid is highly corrosive. Operators should wear labor protection equipment and maintain good ventilation in the workshop.
Storage method
Store in a dry and ventilated place, protected from sun and moisture. Store and transport in accordance with the general regulations for toxic chemicals.
Synthesis method
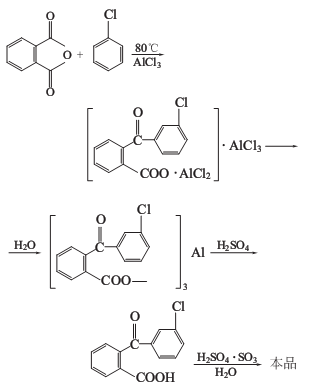
Phthalic anhydride and chlorobenzene react in the presence of anhydrous aluminum trichloride to obtain chlorobenzoyl aluminum benzoate double salt, which is hydrolyzed and acidified, and then smoked Sulfuric acid is dehydrated, loop closed, separated and filtered to obtain the finished product.
Purpose
Used as an important dye intermediate. It is mainly used to produce 2-aminoanthraquinone and further produce vat dyes, such as vat blue RSN, vat blue BC, etc. It can also be used to make alizarin.



 微信扫一扫打赏
微信扫一扫打赏
