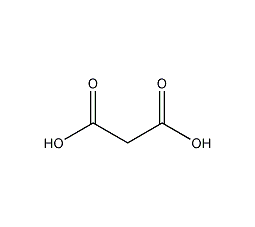
Structural formula
| Business number | 03UC |
|---|---|
| Molecular formula | C3H4O4 |
| Molecular weight | 104 |
| label |
propanedicarboxylic acid, Carotene acid, Methanedicarboxylic acid, betaine acid, malic acid, Propanedioic acid, Methane dicarboxylic acid, Aliphatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:141-82-2
MDL number:MFCD00002707
EINECS number:205-503-0
RTECS number:OO0175000
BRN number:1751370
PubChem ID:None
Physical property data
1. Physical property data:
1. Shape: white crystal.
2. Flash point (ºC): 157
3. Properties: colorless fine crystals
4. Relative density (d20
sup>4) : 1.546
5. Melting point (ºC): 134~135 (dec)
6. Boiling point (ºC, normal pressure): 140 (dec)
7. Relative density (20℃, 4℃): 1.61010
8. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -861.15
9. The crystal phase standard claims heat (enthalpy) (kJ·mol-1): – 891.02
10. Solubility: soluble in water, soluble in ethanol and ether.
Toxicological data
2. Toxicological data:
1. Acute toxicity: Rat oral LDLo: 1310 mg/kg;
Rat intraperitoneal LD50: 1500 mg/kg;
Mouse oral LD50: 4 mg/kg;
Mouse intraperitoneal LD50: 300 mg/kg
Rabbit transdermal LDLo: 10 mg/kg;
2. Chronic toxicity/carcinogenicity
Rat oral TDLo: 10080 mg/kg/6W
3. Inhalation toxicity: Rat: >8900 mg/m3/1H
Ecological data
3. Ecological data:
1. Other harmful effects: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 19.07
2. Molar volume (cm3/mol): 67.3
3 , Isotonic specific volume (90.2K): 195.0
4. Surface tension (dyne/cm): 70.5
p>
5. Polarizability (10-24cm3): 7.56
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 83.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: alkali, oxidizing agent, reducing agent.
2.Low toxicity. It has an irritating effect on the skin and mucous membranes, but is not as severe as oxalic acid. The oral LD50 for mice is 1.54g/kg. Special protection is generally not required when producing malonic acid, but cyanoacetic acid and sodium cyanide are both potent poisons, so special care must be taken when handling compounds containing cyano groups, and protective gear must be worn and corresponding safety measures must be formulated.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves, and mainstream smoke.
4. Can sublimate in vacuum.
Storage method
1. Seal and store.
2. Packed in a woven bag lined with plastic lining, net weight 25kg. Store and transport according to general chemical regulations.
Synthesis method
1. In industry, malonic acid is produced by hydrolyzing cyanoacetic acid or diethyl malonate. Since malonic acid itself is not very stable, it is used in organic synthesis through diethyl malonate. Malonic acid exists in beetroot in the form of calcium salt, and the scale deposited in the retractable tanks for beet sugar production is calcium malonate. Malonic acid is a metabolic intermediate, and its derivatives have biological activity. Malonate esters, especially diethyl malonate, are extremely important intermediates in organic synthesis, just like ethyl acetoacetate. The intermediate for preparing malonic acid and its derivatives is cyanoacetic acid. Cyanoacetic acid is converted into free malonic acid through hydrolysis. Generally, esterification is performed directly without separation. It is usually converted into diethyl ester, while free malonic acid is usually produced by hydrolysis of diethyl malonate.
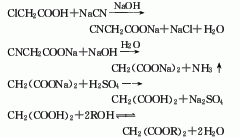
p>
2. The preparation method is to add sodium carbonate aqueous solution to chloroacetic acid in the reaction kettle to form sodium chloroacetate aqueous solution, and then slowly add 30% sodium cyanide solution dropwise at a predetermined temperature. The reaction produces sodium cyanoacetate. After the cyanation reaction is completed, add sodium hydroxide to heat up and hydrolyze to generate sodium malonate solution, which is concentrated, and then dropwise added with sulfuric acid to acidify to generate malonic acid, which is filtered and dried to obtain the product.
3. Tobacco: FC, 54; FC, 50; BU, 26; FC, 38.
Purpose
1. Mainly used in pharmaceutical intermediates, but also in spices, adhesives, resin additives, electroplating polishing agents, etc.
2. Used as complexing agent and also used in the preparation of barbiturate salt.
3. Malonic acid is an intermediate of the fungicide Daphne, and also an intermediate of the plant growth regulator indocyl ester.
4. Malonic acid and its esters are mainly used in spices, adhesives, resin additives, pharmaceutical intermediates, electroplating polishing agents, explosion control agents, thermal welding flux additives, etc. In the pharmaceutical industry, it is used to produce luminal, barbiturates, vitamin B1, vitamin B2, vitamin B6, phenylphenylbutazone, amino acids, etc. Malonic acid is used as an aluminum surface treatment agent. Since it only generates water and carbon dioxide when heated and decomposed, there is no pollution problem. At this point, it has great advantages compared with acid-type treatment agents such as formic acid used in the past.
5.Malonic acid is used as an additive substance in electroless plating and also as an electroplating polishing agent.



 微信扫一扫打赏
微信扫一扫打赏
