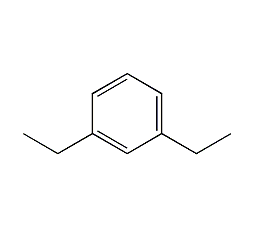
Structural formula
| Business number | 03UJ |
|---|---|
| Molecular formula | C10H14 |
| Molecular weight | 134.22 |
| label |
1,3-diethylbenzene, 1,3-Diethyl benzene, Cross-linking agent for polystyrene resin, high boiling point solvents, For organic synthesis |
Numbering system
CAS number:141-93-5
MDL number:MFCD00009260
EINECS number:205-511-4
RTECS number:CZ5620000
BRN number:1903394
PubChem number:24890191
Physical property data
1. Properties: colorless liquid with aromatic odor. [1]
2. Melting point (℃): -83.9[2]
3. Boiling point (℃): 181.1[3]
4. Relative density (water = 1): 0.86[4]
5. Relative vapor Density (air=1): 4.6[5]
6. Saturated vapor pressure (kPa): 1.33 (61.4℃)[6]
7. Critical temperature (℃): 389.8[7]
8. Critical pressure (MPa): 2.88[8]
9. Octanol/water partition coefficient: 4.44[9]
10. Flash point (℃): 56[10]
11. Ignition temperature (℃): 430[11]
12. Solubility: insoluble in water, soluble in ethanol , ether, benzene, carbon tetrachloride and most other organic solvents. [12]
13. Flash point (ºC): 450
14. Heat of evaporation (KJ/mol, 25ºC): 52.54
15. Heat of fusion (KJ/mol): 10.97
16. Heat of formation (KJ/mol, 298K, gas): 21.86
17. Specific heat capacity (KJ/(kg ·K), 298K, constant pressure): 1.32
18. Relative density (20℃, 4℃): 0.8639
19. Refractive index at room temperature (n25): 1.4931
20. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5913.54
21. Gas phase standard Claimed heat (enthalpy) (kJ·mol-1): -22.38
22. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1 sup>): -5862.41
23. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -73.51
24. Liquid Phase standard hot melt (J·mol-1·K-1): 248.7
Toxicological data
1. Acute toxicity[13] LD50: 1200mg/kg (rat oral)
2. Irritation No data available
3. Subacute and chronic toxicity[14] Rats, guinea pigs, rabbits and monkeys inhaled 2.61~9.5g/m3 for 7 hours a day for a total of 103~138 days. Mild pathological changes were found in the liver, kidneys and testicles.
Ecological data
1. Ecotoxicity[15] LC50: 4.5mg/L (96h) (fathead minnow, dynamic); 3.97mg /L (96h) (Medaka)
2. Biodegradability No data available
3. Non-biodegradability [16] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 1d (theoretical).
4. Bioconcentration[17] BCF: 520 (theoretical); 350~854 (carp, Exposure concentration 20mg/L, exposure time 6 weeks); 413~653 (carp, exposure concentration 2mg/L, exposure time 6 weeks)
5. Other harmful effects [18] This substance is harmful to the environment and special attention should be paid to aquatic biological systems. Bioaccumulation occurs especially in fish. But once out of the polluted water body, the pollutants in the fish are discharged relatively quickly.
Molecular structure data
1. Molar refractive index: 45.35
2. Molar volume (cm3/mol): 155.0
3. Isotonic specific volume (90.2K ): 360.4
4. Surface tension (dyne/cm): 29.1
5. Polarizability (10-24cm3): 17.97
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 76
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stability
2. Incompatible substances [20] Strong oxidants, acids, halogens, etc.
3. Polymerization hazards[21] No aggregation
Storage method
Storage Precautions[22] Stored in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
None yet
Purpose
1. Mainly used in the production of divinylbenzene.
2. Used as solvent and organic synthesis intermediate. [23]



 微信扫一扫打赏
微信扫一扫打赏
