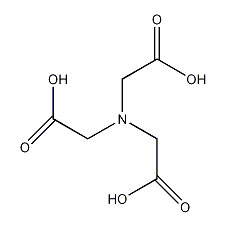
Structural formula
| Business number | 03SA |
|---|---|
| Molecular formula | C6H9NO6 |
| Molecular weight | 191.14 |
| label |
Trilon A; aminocarboxylic complexing agent I, Nitrilotriacetic acid; Nitrilotriacetic acid, Ammonium triacetate;tricarbonylmethylamine, Iminotriacetic acid, N,N-Bis(carboxymethyl)glycine, NTA, Tris(carboxymethyl)amine, Complexone I, Aminotriacetic acid, Trilon A, aliphatic compounds |
Numbering system
CAS number:139-13-9
MDL number:MFCD00004287
EINECS number:205-355-7
RTECS number:AJ0175000
BRN number:1710776
PubChem number:24897916
Physical property data
1. Physical property data
1. Character: white rhombus crystal.
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 241.5
5. Boiling point (ºC, 0.67kpa or 5 mmHg): Uncertain
6. Refractive index: Uncertain
p>
7. Flash point (ºC): Uncertain
8. Specific rotation (ºC): Uncertain
9. Autoignition point or ignition temperature (ºC ) Uncertain
10. Vapor pressure (kPa, 25ºC): Uncertain
11. Saturated vapor pressure (kPa, 60ºC): Uncertain
12 . Heat of combustion (KJ/mol): Uncertain
13. Critical temperature (ºC): 50.3
14. Critical pressure (KPa): Uncertain
15. Log value of oil-water (octanol/water) partition coefficient: Uncertain
16. Explosion upper limit (%, V/V): Uncertain
17. Explosion lower limit (%, V/V): Uncertain
18. Solubility: Slightly soluble in hot water, soluble in ammonia water and sodium hydroxide solution. At 22.5°C, 1L of water can dissolve 1.28g of aminotriacetic acid, and the ph value of the saturated aqueous solution is 2.3. Insoluble in most organic solutions.
Toxicological data
Acute toxicity data:
Rat oral LD50: 1100mg/kg
Mouse oral LC50: 3160mg/kg
Mouse intraperitoneal LC50 : 325mg/kg
Other multiple dose data;
Rat oral TDLo: 41292mg/kg/7W-C
Tumorigenic data:
Oral TDLo in rats: 430mg/kg/75W-C
Oral TCLo in mice: 832mg/kg/66W-C
CauseMutation data:
Morphological changes in hamster embryos: 2mg/L
Ecological data
None
Molecular structure data
Molecular property data:
1. Molar refractive index: 38.26
2. Molar volume (cm3/mol): 118.7
3. Isotonic specific volume (90.2K): 361.9
4. Surface tension (3.0 dyne/cm): 86.4
5. Polarizability (0.5 10-24cm3): 15.16
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -3.2
2. Number of hydrogen bond donors: 3
3. Number of hydrogen bond acceptors: 7
4. Number of rotatable chemical bonds: 6
5. Number of tautomers: none
6. Topological molecule polar surface area 115
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 187
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is flammable and may cause combustion when exposed to open flames, high heat, or oxidants.
2.Can form complexes with various metal ions.
Storage method
Should be kept sealed
Synthesis method
1. Chloroacetic acid reacts with sodium hydroxide to produce sodium chloroacetate, and then ammonium chloride reacts to produce sodium nitrilotriacetate, which is then acidified to obtain the finished product. The chloroacetic acid method has backward technology and high cost, and has been eliminated abroad. Manufacturers of a large amount of by-product HCN in the United States and Japan use the Strigor method. Pure liquid HCN and formaldehyde react with ammonia or amine salts under acidic conditions to form nitrogen triacetonitrile, which is then hydrolyzed to obtain NTA. This method has high investment and operation costs and poor safety. Another method is the carboxymethylation method successfully developed abroad in the 1970s. This method uses NaCN, which is convenient for storage and transportation. The reaction is carried out under alkaline conditions, does not produce volatile HCN, and has good production safety.
2.The reaction between chloroacetic acid and sodium hydroxide produces sodium chloroacetate, which then reacts with ammonium chloride to produce sodium nitrilotriacetate, which is then acidified The finished product is obtained.
3.Dissolve glycine and iodoacetic acid in water, add potassium hydroxide solution, heat to 80°C, cool, and then use hydrochloric acid After acidification, iminotriacetic acid precipitates, and the pure product is obtained by recrystallizing in hot water:
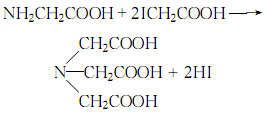
4.Put 885kg of monochloroacetic acid into the acid-resistant kettle and heat to 64°C with stirring. After complete melting, 1220kg of 30% liquid caustic soda is added dropwise for neutralization reaction. React at 80~90℃ for 5~6h. An aqueous sodium monochloroacetate solution was obtained. The neutralized liquid is sent to a thin film evaporator and concentrated under vacuum. When it is close to saturation, pour it into the condensation kettle, heat it to 60°C, and adjust the pH value to 7-8 with saturated sodium carbonate solution. Continue to raise the temperature to 80°C, and add 400kg of saturated ammonium chloride solution while stirring. After adding, stir for another 5 minutes and mix evenly. Then use liquid caustic soda to adjust the pH value to 9-10. Stop reacting. Let stand for 4h. filter. Collect the filtrate. Pour the filtrate into the acidification kettle and add hydrochloric acid to acidify it. When the pH value reaches 0.5 to 1.2, stop adding acid. Cool and let stand to crystallize. The supernatant liquid is sucked out, the crystals are washed with water several times, and dried to obtain the finished product. The reaction formula is as follows:
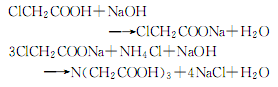
Purpose
1. Complexing agent It is a well-known complexing agent that can form metal complexes with a variety of metals for analysis and measurement, and separate rare metals. It can also be used to extract individual metals from rare metals.
2. Catalyst In the production of polyurethane foam, it can be used as a foaming catalyst, which can foam immediately and gel quickly.
3. Stabilizer It can be used as a stabilizer in polystyrene production.
4. Detergent is used in the steam generator system of nuclear reactors. It can be injected into the steam-water cycle as an aqueous solution to remove scale and corrosive substances between the walls. It does not affect the efficiency during the addition process. .
5. Plating agent In non-toxic electroplating, it can speed up the deposition speed.
6. Color photographic developer is one of the important components in color photographic development and can be used to prevent precipitation during development. Nitrilotriacetic acid is a substitute for tripolyphosphate and has also received attention in solving the problem of eutrophication of water bodies caused by synthetic detergents.
7. Nitrilotriacetic acid is an organic polycarboxylic acid chelating agent. Because of its small molecule, it can chelate more metals and Strong biodegradability. Adding a certain amount of reducing agent and pH regulator to nitrilotriacetic acid has excellent effect in removing Fe2O3 and hematite oxides.
8. Used as analytical reagents, such as coordinating titrants or cooperating masking agents. Also used in color development, printing and dyeing and non-toxic electroplating.
9.As a metal ion chelating agent, it is widely used in textile, papermaking and other industries.
��Metal ion chelating agents are widely used in textile, papermaking and other industries.



 微信扫一扫打赏
微信扫一扫打赏
